Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice
- PMID: 32594056
- PMCID: PMC7749468
- DOI: 10.4103/1673-5374.285001
Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice
Abstract
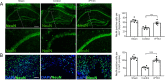
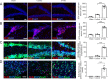
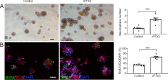
Emerging evidence indicates that pentraxin 3 is an acute-phase protein that is linked with the immune response to inflammation. It is also a newly discovered marker of anti-inflammatory A2 reactive astrocytes, and potentially has multiple protective effects in stroke; however, its role in the adult brain after traumatic brain injury is unknown. In the present study, a moderate model of traumatic brain injury in mice was established using controlled cortical impact. The models were intraventricularly injected with recombinant pentraxin 3 (the recombinant pentraxin 3 group) or an equal volume of vehicle (the control group). The sham-operated mice underwent craniotomy, but did not undergo the controlled cortical impact. The potential neuroprotective and neuroregenerative roles of pentraxin 3 were investigated on days 14 and 21 after traumatic brain injury. Western blot assay showed that the expression of endogenous pentraxin 3 was increased after traumatic brain injury in mice. Furthermore, the neurological severity test and wire grip test revealed that recombinant pentraxin 3 treatment reduced the neurological severity score and increased the wire grip score, suggesting an improved recovery of sensory-motor functions. The Morris water maze results demonstrated that recombinant pentraxin 3 treatment reduced the latency to the platform, increased the time spent in the correct quadrant, and increased the number of times traveled across the platform, thus suggesting an improved recovery of cognitive function. In addition, to investigate the effects of pentraxin 3 on astrocytes, specific markers of A2 astrocytes were detected in primary astrocyte cultures in vitro using western blot assay. The results demonstrated that pentraxin 3 administration activates A2 astrocytes. To explore the protective mechanisms of pentraxin 3, immunofluorescence staining was used. Intraventricular injection of recombinant pentraxin 3 increased neuronal maintenance in the peri-injured cortex and ipsilateral hippocampus, increased the number of doublecortin-positive neural progenitor cells in the subventricular and subgranular zones, and increased the number of bromodeoxyuridine (proliferation) and neuronal nuclear antigen (mature neuron) double-labeled cells in the hippocampus and peri-injured cortex. Pentraxin 3 administration also increased the number of neurospheres and the number of bromodeoxyuridine and doublecortin double-labeled cells in neurospheres, and enhanced the proliferation of neural progenitor cells in primary neural progenitor cell cultures in vitro. In conclusion, recombinant pentraxin 3 administration activated A2 astrocytes, and consequently improved the recovery of neural function by increasing neuronal survival and enhancing neurogenesis. All experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, China on March 1, 2016.
Keywords: brain injury; brain trauma; cells; neurogenesis; plasticity; protein; recovery; regeneration.
Conflict of interest statement
None
Figures



References
-
- Bermpohl D, You Z, Korsmeyer SJ, Moskowitz MA, Whalen MJ. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab. 2006;26:625–633. - PubMed
LinkOut - more resources
Full Text Sources

