Rectal Omeprazole in Infants With Gastroesophageal Reflux Disease: A Randomized Pilot Trial
- PMID: 32594305
- PMCID: PMC7511285
- DOI: 10.1007/s13318-020-00630-8
Rectal Omeprazole in Infants With Gastroesophageal Reflux Disease: A Randomized Pilot Trial
Abstract
Background and objective: Omeprazole is a proton pump inhibitor that is used in acid suppression therapy in infants. Infants cannot swallow the oral tablets or capsules. Since, infants require a non-standard dose of omeprazole, the granules or tablets are often crushed or suspended in water or sodium bicarbonate, which may destroy the enteric coating. In this study we explore the efficacy and pharmacokinetics of rectally administered omeprazole in infants with gastroesophageal reflux disease (GERD) due to esophageal atresia (EA) or congenital diaphragmatic hernia (CDH) and compare these with orally administered omeprazole.
Methods: Infants (6-12 weeks postnatal and bodyweight > 3 kg) with EA or CDH and GERD were randomized to receive a single dose of 1 mg/kg omeprazole rectally or orally. The primary outcome was the percentage of infants for whom omeprazole was effective according to predefined criteria for 24-h intraesophageal pH. Secondary outcomes were the percentages of time that gastric pH was < 3 or < 4, as well as the pharmacokinetic parameters.
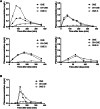
Results: Seventeen infants, 4 with EA and 13 with CDH, were included. The proportion of infants for whom omeprazole was effective was 56% (5 of 9 infants) after rectal administration and 50% (4 of 8 infants) after oral administration. The total reflux time in minutes and percentages and the number of reflux episodes of pH < 4 decreased statistically significantly after both rectal and oral omeprazole administration. Rectal and oral administration of omeprazole resulted in similar serum exposure.
Conclusions: A single rectal omeprazole dose (1 mg/kg) results in consistent increases in intraesophageal and gastric pH in infants with EA- or CDH-related GERD, similar to an oral dose. Considering the challenges with existing oral formulations, rectal omeprazole presents as an innovative, promising alternative for infants with pathological GERD.
Clinical trial register: ClinicalTrials.gov Identifier: NCT00226044.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Vandenplas Y, Rudolph CD, DiLorenzo C, et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical