Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial
- PMID: 32594522
- PMCID: PMC7983954
- DOI: 10.1111/bjd.19341
Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial
Abstract
Background: Patients with plaque psoriasis treated with biologic therapies need more efficacious, safe and convenient treatments to improve quality of life. Risankizumab and secukinumab inhibit interleukin-23 and interleukin-17A, respectively, and are effective in adult patients with moderate-to-severe plaque psoriasis but have different dosing regimens.
Objectives: To compare directly the efficacy and safety of risankizumab vs. secukinumab over 52 weeks.
Methods: IMMerge was an international, phase III, multicentre, open-label, efficacy-assessor-blinded, active-comparator study, in which adult patients with chronic, moderate-to-severe plaque psoriasis were randomized in a 1 : 1 ratio to treatment with risankizumab 150 mg or secukinumab 300 mg. Primary efficacy endpoints were the proportions of patients achieving ≥ 90% improvement from baseline in Psoriasis Area and Severity Index (PASI 90) at week 16 (noninferiority comparison with margin of 12%) and week 52 (superiority comparison).
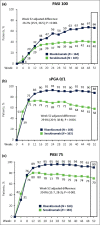
Results: In total 327 patients from nine countries were treated with risankizumab (n = 164) or secukinumab (n = 163). Risankizumab was noninferior to secukinumab in the proportion of patients achieving PASI 90 at week 16 [73·8% vs. 65·6%; difference of 8·2%, 96·25% confidence interval (CI)-2·2 to 18·6; within the 12% noninferiority margin] and superior to secukinumab at week 52 (86·6% vs. 57·1%; difference of 29·8%, 95% CI 20·8-38·8; P < 0·001), thus meeting both primary endpoints. All secondary endpoints (PASI 100, static Physician's Global Assessment 0 or 1, and PASI 75) at week 52 demonstrated superiority for risankizumab vs. secukinumab (P < 0·001). No new safety concerns were identified.
Conclusions: At week 52, risankizumab demonstrated superior efficacy and similar safety with less frequent dosing compared with secukinumab.
© 2020 British Association of Dermatologists.
Figures


Comment in
-
Complete skin clearance and beyond.Br J Dermatol. 2021 Jan;184(1):3-4. doi: 10.1111/bjd.19544. Epub 2020 Oct 8. Br J Dermatol. 2021. PMID: 33029797 Free PMC article.
References
-
- Parisi R, Symmons DPM, Griffiths CEM et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–85. - PubMed
-
- Lebwohl MG, Bachelez H, Barker J et al. Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70:871–81. - PubMed
-
- Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun 2010; 34:J314–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

