Arylphosphonate-Directed Ortho C-H Borylation: Rapid Entry into Highly-Substituted Phosphoarenes
- PMID: 32594742
- PMCID: PMC7405988
- DOI: 10.1021/jacs.0c04159
Arylphosphonate-Directed Ortho C-H Borylation: Rapid Entry into Highly-Substituted Phosphoarenes
Abstract
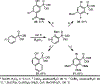
Phosphonate-directed ortho C-H borylation of aromatic phosphonates is reported. Using simple starting materials and commercially accessible catalysts, this method provides steady access to o-phosphonate arylboronic esters bearing pendant functionality and flexible substitution patterns. These products serve as flexible precursors for a variety of highly substituted phosphoarenes, and in situ downstream functionalization of the products is described.
Figures


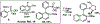
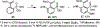
References
-
- Quin LD, A Guide to Organophosphorus Chemistry. Wiley-Interscience: New York, 2000.
-
- Iaroshenko V, Organophosphorus Chemistry: From Molecules to Applications. Wiley-VCH: Weinheim, 2019.
-
- Beugelmans R; Bois-Choussy M; Gayral P; Rigothier MC, Synthèse par SRN1 et évaluation de l’activité amoebicide de nouveaux dérivés quinoléiniques. Eur. J. Med. Chem 1988, 23, 539–546, 10.1016/0223-5234(88)90097-9. - DOI
-
- Veale CA; Damewood JR Jr.; Steelman GB; Bryant C; Gomes B; Williams J, Non-peptidic Inhibitors of Human Leukocyte Elastase. 4. Design, Synthesis, and in Vitro and in Vivo Activity of a Series of β-Carbolinone-Containing Trifluoromethyl Ketones. J. Med. Chem 1995, 38, 86–97, 10.1021/jm00001a014. - DOI - PubMed
-
- Ortwine DF; Malone TC; Bigge CF; Drummond JT; Humblet C; Johnson G; Pinter GW, Generation of N-Methyl-D-aspartate Agonist and Competitive Antagonist Pharmacophore Models. Design and Synthesis of Phosphonoalkyl-Substituted Tetrahydroisoquinolines as Novel Antagonists. J. Med. Chem 1992, 35, 1345–1370, 10.1021/jm00086a004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

