Neuronal Networks in Hypertension: Recent Advances
- PMID: 32594802
- PMCID: PMC7347452
- DOI: 10.1161/HYPERTENSIONAHA.120.14521
Neuronal Networks in Hypertension: Recent Advances
Abstract
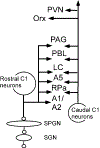
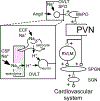
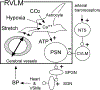
Neurogenic hypertension is associated with excessive sympathetic nerve activity to the kidneys and portions of the cardiovascular system. Here we examine the brain regions that cause heightened sympathetic nerve activity in animal models of neurogenic hypertension, and we discuss the triggers responsible for the changes in neuronal activity within these regions. We highlight the limitations of the evidence and, whenever possible, we briefly address the pertinence of the findings to human hypertension. The arterial baroreflex reduces arterial blood pressure variability and contributes to the arterial blood pressure set point. This set point can also be elevated by a newly described cerebral blood flow-dependent and astrocyte-mediated sympathetic reflex. Both reflexes converge on the presympathetic neurons of the rostral medulla oblongata, and both are plausible causes of neurogenic hypertension. Sensory afferent dysfunction (reduced baroreceptor activity, increased renal, or carotid body afferent) contributes to many forms of neurogenic hypertension. Neurogenic hypertension can also result from activation of brain nuclei or sensory afferents by excess circulating hormones (leptin, insulin, Ang II [angiotensin II]) or sodium. Leptin raises blood vessel sympathetic nerve activity by activating the carotid bodies and subsets of arcuate neurons. Ang II works in the lamina terminalis and probably throughout the brain stem and hypothalamus. Sodium is sensed primarily in the lamina terminalis. Regardless of its cause, the excess sympathetic nerve activity is mediated to some extent by activation of presympathetic neurons located in the rostral ventrolateral medulla or the paraventricular nucleus of the hypothalamus. Increased activity of the orexinergic neurons also contributes to hypertension in selected models.
Keywords: baroreflex; carotid body; hypothalamus; leptin; medulla oblongata.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

