Therapy-related myelodysplastic syndromes deserve specific diagnostic sub-classification and risk-stratification-an approach to classification of patients with t-MDS
- PMID: 32595214
- PMCID: PMC7932916
- DOI: 10.1038/s41375-020-0917-7
Therapy-related myelodysplastic syndromes deserve specific diagnostic sub-classification and risk-stratification-an approach to classification of patients with t-MDS
Abstract
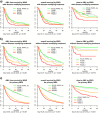
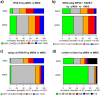
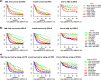
In the current World Health Organization (WHO)-classification, therapy-related myelodysplastic syndromes (t-MDS) are categorized together with therapy-related acute myeloid leukemia (AML) and t-myelodysplastic/myeloproliferative neoplasms into one subgroup independent of morphologic or prognostic features. Analyzing data of 2087 t-MDS patients from different international MDS groups to evaluate classification and prognostication tools we found that applying the WHO classification for p-MDS successfully predicts time to transformation and survival (both p < 0.001). The results regarding carefully reviewed cytogenetic data, classifications, and prognostic scores confirmed that t-MDS are similarly heterogeneous as p-MDS and therefore deserve the same careful differentiation regarding risk. As reference, these results were compared with 4593 primary MDS (p-MDS) patients represented in the International Working Group for Prognosis in MDS database (IWG-PM). Although a less favorable clinical outcome occurred in each t-MDS subset compared with p-MDS subgroups, FAB and WHO-classification, IPSS-R, and WPSS-R separated t-MDS patients into differing risk groups effectively, indicating that all established risk factors for p-MDS maintained relevance in t-MDS, with cytogenetic features having enhanced predictive power. These data strongly argue to classify t-MDS as a separate entity distinct from other WHO-classified t-myeloid neoplasms, which would enhance treatment decisions and facilitate the inclusion of t-MDS patients into clinical studies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds). WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

