Inhibition of Adenosine Pathway Alters Atrial Electrophysiology and Prevents Atrial Fibrillation
- PMID: 32595514
- PMCID: PMC7304385
- DOI: 10.3389/fphys.2020.00493
Inhibition of Adenosine Pathway Alters Atrial Electrophysiology and Prevents Atrial Fibrillation
Abstract
Background: Adenosine leads to atrial action potential (AP) shortening through activation of adenosine 1 receptors (A1-R) and subsequent opening of G-protein-coupled inwardly rectifying K+ channels. Extracellular production of adenosine is drastically increased during stress and ischemia.
Objective: The aim of this study was to address whether the pharmacological blockade of endogenous production of adenosine and of its signaling prevents atrial fibrillation (AF).
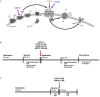
Methods: The role of A1-R activation on atrial action potential duration, refractoriness, and AF vulnerability was investigated in rat isolated beating heart preparations (Langendorff) with an A1-R agonist [2-chloro-N 6-cyclopentyladenosine (CCPA), 50 nM] and antagonist [1-butyl-3-(3-hydroxypropyl)-8-(3-noradamantyl)xanthine (PSB36), 40 nM]. Furthermore, to interfere with the endogenous adenosine release, the ecto-5'-nucleotidase (CD73) inhibitor was applied [5'-(α,β-methylene) diphosphate sodium salt (AMPCP), 500 μM]. Isolated trabeculae from human right atrial appendages (hRAAs) were used for comparison.
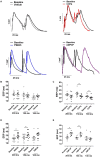
Results: As expected, CCPA shortened AP duration at 90% of repolarization (APD90) and effective refractory period (ERP) in rat atria. PSB36 prolonged APD90 and ERP in rat atria, and CD73 inhibition with AMPCP prolonged ERP in rats, confirming that endogenously produced amount of adenosine is sufficiently high to alter atrial electrophysiology. In human atrial appendages, CCPA shortened APD90, while PSB36 prolonged it. Rat hearts treated with CCPA are prone to AF. In contrast, PSB36 and AMPCP prevented AF events and reduced AF duration (vehicle, 11.5 ± 2.6 s; CCPA, 40.6 ± 16.1 s; PSB36, 6.5 ± 3.7 s; AMPCP, 3.0 ± 1.4 s; P < 0.0001).
Conclusion: A1-R activation by intrinsic adenosine release alters atrial electrophysiology and promotes AF. Inhibition of adenosine pathway protects atria from arrhythmic events.
Keywords: A1-R; CD73; adenosine; arrhythmias; hypoxia; translational models.
Copyright © 2020 Soattin, Lubberding, Bentzen, Christ and Jespersen.
Figures




References
-
- Belardinelli L., Isenberg G. (1983). Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am. J. Physiol. 244 H734–H737. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

