To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host
- PMID: 32595652
- PMCID: PMC7304505
- DOI: 10.3389/fimmu.2020.01250
To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host
Erratum in
-
Corrigendum: To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host.Front Immunol. 2021 Oct 27;12:780758. doi: 10.3389/fimmu.2021.780758. eCollection 2021. Front Immunol. 2021. PMID: 34777397 Free PMC article.
Abstract
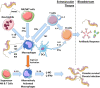
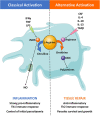
African trypanosomes are single-celled extracellular protozoan parasites transmitted by tsetse fly vectors across sub-Saharan Africa, causing serious disease in both humans and animals. Mammalian infections begin when the tsetse fly penetrates the skin in order to take a blood meal, depositing trypanosomes into the dermal layer. Similarly, onward transmission occurs when differentiated and insect pre-adapted forms are ingested by the fly during a blood meal. Between these transmission steps, trypanosomes access the systemic circulation of the vertebrate host via the skin-draining lymph nodes, disseminating into multiple tissues and organs, and establishing chronic, and long-lasting infections. However, most studies of the immunobiology of African trypanosomes have been conducted under experimental conditions that bypass the skin as a route for systemic dissemination (typically via intraperitoneal or intravenous routes). Therefore, the importance of these initial interactions between trypanosomes and the skin at the site of initial infection, and the implications for these processes in infection establishment, have largely been overlooked. Recent studies have also demonstrated active and complex interactions between the mammalian host and trypanosomes in the skin during initial infection and revealed the skin as an overlooked anatomical reservoir for transmission. This highlights the importance of this organ when investigating the biology of trypanosome infections and the associated immune responses at the initial site of infection. Here, we review the mechanisms involved in establishing African trypanosome infections and potential of the skin as a reservoir, the role of innate immune cells in the skin during initial infection, and the subsequent immune interactions as the parasites migrate from the skin. We suggest that a thorough identification of the mechanisms involved in establishing African trypanosome infections in the skin and their progression through the host is essential for the development of novel approaches to interrupt disease transmission and control these important diseases.
Keywords: African trypanosomiasis; Trypanosoma brucei; innate immunity; neglected tropical disease; skin; transmission.
Copyright © 2020 Alfituri, Quintana, MacLeod, Garside, Benson, Brewer, Mabbott, Morrison and Capewell.
Figures

References
-
- de Raadt P. The history of sleeping sickness. In: Fourth International Course on African Trypanosomiasis. (2005)
Publication types
MeSH terms
Grants and funding
- BBS/E/D/20002174/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 209511/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- BB/J01446X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20231762 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

