Alkene-Linked Covalent Organic Frameworks Boosting Photocatalytic Hydrogen Evolution by Efficient Charge Separation and Transfer in the Presence of Sacrificial Electron Donors
- PMID: 32596107
- PMCID: PMC7312270
- DOI: 10.1002/advs.201902988
Alkene-Linked Covalent Organic Frameworks Boosting Photocatalytic Hydrogen Evolution by Efficient Charge Separation and Transfer in the Presence of Sacrificial Electron Donors
Abstract
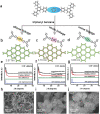
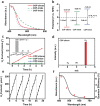
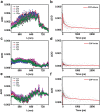
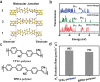
Covalent organic frameworks (COFs) are potential photocatalysts for artificial photosynthesis but they are much less explored for photocatalytic hydrogen evolution (PHE). COFs, while intriguing due to crystallinity, tunability, and porosity, tend to have low apparent quantum efficiency (AQE) and little is explored on atomistic structure-performance correlation. Here, adopting triphenylbenzene knots and phenyl linkers as a proof of concept, three structurally related COFs with different linkages are constructed to achieve a tunable COF platform and probe the effect of the linkage chemistry on PHE. Cyano-substituted alkene-linked COF (COF-alkene) yields a stable 2330 µmol h-1 g-1 PHE rate, much superior to imine- and imide-linked counterparts (<40 µmol h-1 g-1) under visible light irradiation. Impressively, COF-alkene achieves an AQE of 6.7% at 420 nm. Combined femtosecond transient absorption spectroscopy and theoretical calculation disclose the critical role of cyano-substituted alkene linkages toward high efficiency of charge separation and transfer in the presence of sacrificial electron donors-the decisive key to the superior PHE performance. Such alkene linkages can also be extended to design a series of high-performance polymeric photocatalysts, highlighting a general design idea for efficient PHE.
Keywords: alkene linkages; charge separation; charge transfer; covalent organic frameworks; photocatalysis.
© 2020 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- a) Wang L., Wan Y. Y., Ding Y. J., Wu S. K., Zhang Y., Zhang X. L., Zhang G. Q., Xiong Y. J., Wu X. J., Yang J. L., Xu H. X., Adv. Mater. 2017, 29, 1702428;
- b) Wang L., Zheng X. S., Chen L., Xiong Y. J., Xu H. X., Angew. Chem., Int. Ed. 2018, 57, 3454. - PubMed
LinkOut - more resources
Full Text Sources
