Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses
- PMID: 32596144
- PMCID: PMC7303270
- DOI: 10.3389/fonc.2020.00819
Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses
Abstract
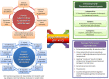
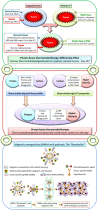
Moderate hyperthermia at temperatures between 40 and 44°C is a multifaceted therapeutic modality. It is a potent radiosensitizer, interacts favorably with a host of chemotherapeutic agents, and, in combination with radiotherapy, enforces immunomodulation akin to "in situ tumor vaccination." By sensitizing hypoxic tumor cells and inhibiting repair of radiotherapy-induced DNA damage, the properties of hyperthermia delivered together with photons might provide a tumor-selective therapeutic advantage analogous to high linear energy transfer (LET) neutrons, but with less normal tissue toxicity. Furthermore, the high LET attributes of hyperthermia thermoradiobiologically are likely to enhance low LET protons; thus, proton thermoradiotherapy would mimic 12C ion therapy. Hyperthermia with radiotherapy and/or chemotherapy substantially improves therapeutic outcomes without enhancing normal tissue morbidities, yielding level I evidence reported in several randomized clinical trials, systematic reviews, and meta-analyses for various tumor sites. Technological advancements in hyperthermia delivery, advancements in hyperthermia treatment planning, online invasive and non-invasive MR-guided thermometry, and adherence to quality assurance guidelines have ensured safe and effective delivery of hyperthermia to the target region. Novel biological modeling permits integration of hyperthermia and radiotherapy treatment plans. Further, hyperthermia along with immune checkpoint inhibitors and DNA damage repair inhibitors could further augment the therapeutic efficacy resulting in synthetic lethality. Additionally, hyperthermia induced by magnetic nanoparticles coupled to selective payloads, namely, tumor-specific radiotheranostics (for both tumor imaging and radionuclide therapy), chemotherapeutic drugs, immunotherapeutic agents, and gene silencing, could provide a comprehensive tumor-specific theranostic modality akin to "magic (nano)bullets." To get a realistic overview of the strength (S), weakness (W), opportunities (O), and threats (T) of hyperthermia, a SWOT analysis has been undertaken. Additionally, a TOWS analysis categorizes future strategies to facilitate further integration of hyperthermia with the current treatment modalities. These could gainfully accomplish a safe, versatile, and cost-effective enhancement of the existing therapeutic armamentarium to improve outcomes in clinical oncology.
Keywords: SWOT analysis; chemotherapy; clinical trials; hyperthermia; hyperthermia treatment planning; immunotherapy; radiation therapy; radiosensitizer.
Copyright © 2020 Datta, Kok, Crezee, Gaipl and Bodis.
Figures







References
-
- Breasted J. The Edwin Schmid surgical papyrus. In: Licht S. editor. Therapeutic Heat and Coal. 2nd ed Baltimore: Waverly Press; (1930). p. 196.
-
- Overgaard J. (editor). History and heritage–an introduction. In: Hyperthermia Oncology 1984. London; Philadelphia, PA: Taylor & Francis; (1985). p. 3–8.
Publication types
LinkOut - more resources
Full Text Sources
Medical

