Activation of STING by cGAMP Regulates MDSCs to Suppress Tumor Metastasis via Reversing Epithelial-Mesenchymal Transition
- PMID: 32596152
- PMCID: PMC7300176
- DOI: 10.3389/fonc.2020.00896
Activation of STING by cGAMP Regulates MDSCs to Suppress Tumor Metastasis via Reversing Epithelial-Mesenchymal Transition
Abstract
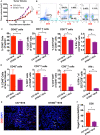
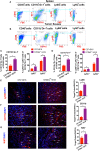
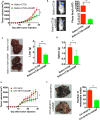
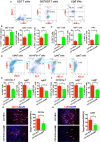
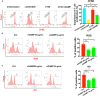
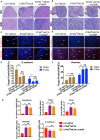
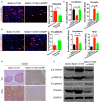
The role of cGAMP stimulating cGAS-cGAMP-STING-IRF3 pathway to inhibit tumor growth was well-established. Herein, the efficiency and pharmacological mechanism of cGAMP on regulating tumor metastasis was investigated. The effects of cGAMP regulating CD8+ T cells and myeloid-derived suppressor cells (MDSCs) in tumor microenvironment was explored. In this study, we found that cGAMP boosted STING signaling pathway to activate the production of IFN-γ from CD8+ T cells, and decreased the population of MDSCs in vivo. The metastasis in CT26 tumor bearing mice was inhibited by cGAMP via regulating EMT process. cGAMP played an important role in suppressing the production of reactive oxygen species (ROS) and nitric oxide (NO) from MDSCs, abolished the suppressive function of MDSCs to the T cells. All in all, the results indicated that the STING agonist cGAMP activated the production of IFN-γ from CD8+ T cells to suppress MDSCs in vivo.
Keywords: STING; cGAMP; epithelial-mesenchymal transition; metastasis; myeloid-derived suppressor cells.
Copyright © 2020 Cheng, Xu, Lu, Yuan, Li, Zhang and Tan.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

