A Guide to Diet-Microbiome Study Design
- PMID: 32596250
- PMCID: PMC7303276
- DOI: 10.3389/fnut.2020.00079
A Guide to Diet-Microbiome Study Design
Abstract
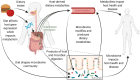
Intense recent interest in understanding how the human gut microbiome influences health has kindled a concomitant interest in linking dietary choices to microbiome variation. Diet is known to be a driver of microbiome variation, and yet the precise mechanisms by which certain dietary components modulate the microbiome, and by which the microbiome produces byproducts and secondary metabolites from dietary components, are not well-understood. Interestingly, despite the influence of diet on the gut microbiome, the majority of microbiome studies published to date contain little or no analysis of dietary intake. Although an increasing number of microbiome studies are now collecting some form of dietary data or even performing diet interventions, there are no clear standards in the microbiome field for how to collect diet data or how to design a diet-microbiome study. In this article, we review the current practices in diet-microbiome analysis and study design and make several recommendations for best practices to provoke broader discussion in the field. We recommend that microbiome studies include multiple consecutive microbiome samples per study timepoint or phase and multiple days of dietary history prior to each microbiome sample whenever feasible. We find evidence that direct effects of diet on the microbiome are likely to be observable within days, while the length of an intervention required for observing microbiome-mediated effects on the host phenotype or host biomarkers, depending on the outcome, may be much longer, on the order of weeks or months. Finally, recent studies demonstrating that diet-microbiome interactions are personalized suggest that diet-microbiome studies should either include longitudinal sampling within individuals to identify personalized responses, or should include an adequate number of participants spanning a range of microbiome types to identify generalized responses.
Keywords: diet; dietary intake; methodology; microbiome; personalized nutrition; study design.
Copyright © 2020 Johnson, Zheng, Kang, Saboe, Knights and Zivkovic.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

