Liquid biopsy mutation panel for non-small cell lung cancer: analytical validation and clinical concordance
- PMID: 32596507
- PMCID: PMC7314769
- DOI: 10.1038/s41698-020-0118-x
Liquid biopsy mutation panel for non-small cell lung cancer: analytical validation and clinical concordance
Abstract
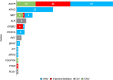
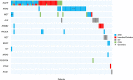
Molecular testing for genomic variants is recommended in advanced non-small cell lung cancer (NSCLC). Standard tissue biopsy is sometimes infeasible, procedurally risky, or insufficient in tumor tissue quantity. We present the analytical validation and concordance study of EGFR variants using a new 17-gene liquid biopsy assay (NCT02762877). Of 144 patients enrolled with newly diagnosed or progressive stage IV nonsquamous NSCLC, 140 (97%) had liquid assay results, and 117 (81%) had both EGFR blood and tissue results. Alterations were detected in 58% of liquid samples. Overall tissue-liquid concordance for EGFR alterations was 94.0% (95% CI 88.1%, 97.6%) with positive percent agreement of 76.7% (57.7%, 90.1%) and negative percent agreement of 100% (95.8%, 100%). Concordance for ALK structural variants was 95.7% (90.1%, 98.6%). This assay detected alterations in other therapeutically relevant genes at a rate similar to tissue analysis. These results demonstrate the analytical and clinical validity of this 17-gene assay.
Keywords: Cancer genomics.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsL.S.S: Consultant for Inivata and Caris Life Sciences. H.H.: None. D.C.: None. S.C.: Received fees from Pfizer for working on their protocols. M.L.T.: None. D.I.: None. C.E.: Consultant fees from Merck Sharp & Dohme, Boehringer Ingelheim, and AstraZeneca; and lecture fees from Bristol-Myers Squibb and Pfizer. J.P.B.: Current employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). K.C.-L.: Current employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). C.S.: Previous employment at Genomic Health, Inc. (now Exact Sciences Corp.) during this work; consultant for Precision Medicine Asia Co Ltd and Cantargia AB. P.T.: None. G.A.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). F.L.B.: Current employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). T.B.: None. A.B.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). J.C.: Reports grants from Genomic Health, during the conduct of the study; personal fees from Genomic Health, outside the submitted work; and Genomic Health provided speakers for educational activities. D.D.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). D.A.E.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). N.G.: None. J.H.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). W.I.,Jr.: None. M.L.: Previous employment at and stock ownership in Genomic Health, Inc. (now Exact Sciences Corp.). J.O.,Jr.: None. B.T.S: None.
Figures
References
-
- Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–306. - PubMed
-
- Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–1515. - PubMed
-
- Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

