Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs
- PMID: 32596545
- PMCID: PMC7310269
- DOI: 10.18063/ijb.v5i2.1.229
Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs
Erratum in
-
ERRATUM.Int J Bioprint. 2020 Sep 17;6(4):309. doi: 10.18063/ijb.v6i4.309. eCollection 2020. Int J Bioprint. 2020. PMID: 33102924 Free PMC article.
Abstract
Bioprinting is increasingly being used for fabrication of engineered tissues for regenerative medicine, drug testing, and other biomedical applications. The success of this technology lies with the development of suitable bioinks and hydrogels that are specific to the intended tissue application. For applications such as neural tissue engineering, conductivity plays an important role in determining the neural differentiation and neural tissue regeneration. Although several conductive hydrogels based on metal nanoparticles (NPs) such as gold and silver, carbon-based materials such as graphene and carbon nanotubes and conducting polymers such as polypyrrole (PPy) and polyaniline were used, they possess several disadvantages. The long-term cytotoxicity of metal nanoparticles (NPs) and carbon-based materials restricts their use in regenerative medicine. The conductive polymers, on the other hand, are non-biodegradable and possess weak mechanical properties limiting their printability into three-dimensional constructs. The aim of this study is to develop a biodegradable, conductive, and printable hydrogel based on collagen and a block copolymer of PPy and polycaprolactone (PCL) (PPy-block-poly(caprolactone) [PPy-b-PCL]) for bioprinting of neural tissue constructs. The printability, including the influence of the printing speed and material flow rate on the printed fiber width; rheological properties; and cytotoxicity of these hydrogels were studied. The results prove that the collagen/PPy-b-PCL hydrogels possessed better printability and biocompatibility. Thus, the collagen/PPy-b-PCL hydrogels reported this study has the potential to be used in the bioprinting of neural tissue constructs for the repair of damaged neural tissues and drug testing or precision medicine applications.
Keywords: Conductive scaffolds; Nerve guide conduit; Peripheral nerve injury; Stem cells; Three-dimensional printing; Tissue engineering scaffolds.
Copyright: © 2019 Vijayavenkataraman S, et al.
Figures


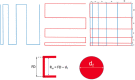



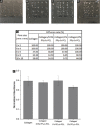
References
-
- Vijayavenkataraman S, Yan WC, Lu WF, et al. 2018, 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Advanced Drug Delivery Reviews. 132:296–332. DOI 10.1016/j.addr.2018.07.004. - PubMed
-
- Merceron TK, Murphy SV. 2015 Hydrogels for 3D Bioprinting Applications, Essentials of 3D Biofabrication and Translation. San Diego: Elsevier; pp. 249–70. DOI 10.1016/b978-0-12-800972-7.00014-1.
-
- Vijayavenkataraman S, Thaharah S, Zhang S, et al. 2018, 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artificial Organs. 43(5):515–23. DOI 10.1111/aor.13360. - PubMed
LinkOut - more resources
Full Text Sources
