Pilot Study of the Biological Properties and Vascularization of 3D Printed Bilayer Skin Grafts
- PMID: 32596551
- PMCID: PMC7294694
- DOI: 10.18063/ijb.v6i1.246
Pilot Study of the Biological Properties and Vascularization of 3D Printed Bilayer Skin Grafts
Erratum in
-
ERRATUM.Int J Bioprint. 2020 Sep 17;6(4):309. doi: 10.18063/ijb.v6i4.309. eCollection 2020. Int J Bioprint. 2020. PMID: 33102924 Free PMC article.
Abstract
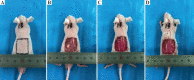
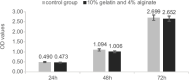
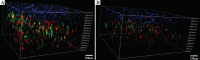
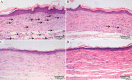
The skin is the largest human organ, and defects in the skin with a diameter greater than 4 cm do not heal without treatment. Allogeneic skin transplantation has been used to allow wound healing, but many grafts do not survive after implantation, due to multiple complications in the procedure. In the present study, the vascularization of three-dimensional (3D) printed full-thickness skin grafts was investigated. Dermal-epithelial grafts were transplanted into a nude mouse model to evaluate integration with the host tissue and the extent of wound healing. To create microvessels in the skin grafts, a bilayer structure consisting of human dermal fibroblasts, keratinocytes, and microvascular endothelial cells was designed and fabricated using an extruded 3D printer. Human dermal fibroblasts and human microvascular endothelial cells were mixed with gelatin-sodium alginate composite hydrogel as the dermis, and human keratinocytes were mixed with gel as the epithelium. Confocal imaging allowed visualization of the location of the cells in the double-layer skin grafts. A full-thickness wound was created on the backs of nude mice and then covered with a double-layer skin graft. Various groups of mice were tested. Animals were euthanized and tissue samples collected after specified time points. Compared with the control group, wound contraction improved by approximately 10%. Histological analysis demonstrated that the new skin had an appearance similar to that of normal skin and with a significant degree of angiogenesis. The results of the immunohistochemical analysis demonstrated that the transplanted cells survived and participated in the healing process.
Keywords: Bilayer skin graft; Gelatin-alginate complex hydrogel; Three-dimensional printing; Vascularization.
Copyright © 2020, Whioce Publishing Pte. Ltd.
Figures





References
-
- Prost-Squarcioni C. Histology of Skin and Hair Follicle. Med Sci (Paris) 2006;22(2):131–7. DOI:10.1051/medsci/2006222131. - PubMed
-
- Bernerd F. Human Skin Reconstructed in vitro as a Model to Study the Keratinocyte, the Fibroblast and Their Interactions:Photodamage and Repair Processes. J Soc Biol. 2005;199(4):313–20. - PubMed
-
- Kim SW, Choi SH, Kim JT, et al. An Additional Option for Split-Thickness Skin Graft Donors:The Previous Free Flap Sites. Ann Plast Surg. 2015;75(6):634–6. DOI:10.1097/SAP.0000000000000143. - PubMed
LinkOut - more resources
Full Text Sources
