Grazing Incidence X-ray Diffraction Studies of Lipid-Peptide Mixed Monolayers during Shear Flow
- PMID: 32596593
- PMCID: PMC7315600
- DOI: 10.1021/acsomega.0c01261
Grazing Incidence X-ray Diffraction Studies of Lipid-Peptide Mixed Monolayers during Shear Flow
Abstract
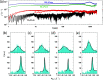
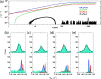
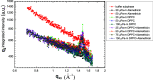
Grazing incidence X-ray diffraction (GIXD) studies of monolayers of biomolecules at an air-water interface give quantitative information of in-plane packing, coherence length of crystalline domains, etc. Rheo-GIXD measurements can reveal quantitative changes in the nanocrystalline domains of a monolayer under shear. Here, we report GIXD studies of monolayers of alamethicin peptide, DPPC lipid, and their mixtures at an air-water interface under steady shear stress. The alamethicin monolayer and the mixed monolayer show a flow jamming transition. On the other hand, the pure 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayer under constant stress flows steadily with a notable enhancement of the area/molecule and coherence lengths, suggesting the fusion of nanocrystallites during flow. The DPPC-alamethicin mixed monolayer shows no significant change in the area/DPPC molecule, but the coherence lengths of the individual phases (DPPC and alamethicin) increase, suggesting that the crystallites of individual phases grow bigger by merging of domains. More phase separation occurs in the system during flow. Our results show that rheo-GIXD has the potential to explore in situ molecular structural changes under rheological conditions for a diverse range of confined biomolecules at interfaces.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Properties of β-sitostanol/DPPC monolayers studied with Grazing Incidence X-ray Diffraction (GIXD) and Brewster Angle Microscopy.J Colloid Interface Sci. 2011 Dec 1;364(1):133-9. doi: 10.1016/j.jcis.2011.08.030. Epub 2011 Aug 19. J Colloid Interface Sci. 2011. PMID: 21903220
-
X-ray grazing incidence diffraction and Langmuir monolayer studies of the interaction of beta-cyclodextrin with model lipid membranes.J Colloid Interface Sci. 2010 Aug 15;348(2):511-21. doi: 10.1016/j.jcis.2010.04.086. Epub 2010 May 4. J Colloid Interface Sci. 2010. PMID: 20493495
-
Protegrin interaction with lipid monolayers: Grazing incidence X-ray diffraction and X-ray reflectivity study.Soft Matter. 2008;4(8):1665-1674. doi: 10.1039/b718295c. Soft Matter. 2008. PMID: 19672319 Free PMC article.
-
Penetration of dissolved amphiphiles into two-dimensional aggregating lipid monolayers.Adv Colloid Interface Sci. 2000 May 24;86(1-2):103-51. doi: 10.1016/s0001-8686(00)00034-8. Adv Colloid Interface Sci. 2000. PMID: 10798352 Review.
-
Grazing incidence X-ray diffraction studies of condensed double-chain phospholipid monolayers formed at the soft air/water interface.Adv Colloid Interface Sci. 2014 May;207:265-79. doi: 10.1016/j.cis.2014.01.005. Epub 2014 Jan 19. Adv Colloid Interface Sci. 2014. PMID: 24507806 Review.
Cited by
-
Comprehensive Approach to the Interpretation of the Electrical Properties of Film-Forming Molecules.J Phys Chem B. 2022 Sep 15;126(36):7037-7046. doi: 10.1021/acs.jpcb.2c04526. Epub 2022 Sep 2. J Phys Chem B. 2022. PMID: 36054662 Free PMC article.
-
The Structure of Oxysterols Determines Their Behavior at Phase Boundaries: Implications for Model Membranes and Structure-Activity Relationships.Adv Exp Med Biol. 2024;1440:3-29. doi: 10.1007/978-3-031-43883-7_1. Adv Exp Med Biol. 2024. PMID: 38036872 Review.
-
X-ray-Based Techniques to Study the Nano-Bio Interface.ACS Nano. 2021 Mar 23;15(3):3754-3807. doi: 10.1021/acsnano.0c09563. Epub 2021 Mar 2. ACS Nano. 2021. PMID: 33650433 Free PMC article.
-
Crystallization of Poly(ethylene)s with Regular Phosphoester Defects Studied at the Air-Water Interface.Polymers (Basel). 2020 Oct 19;12(10):2408. doi: 10.3390/polym12102408. Polymers (Basel). 2020. PMID: 33086637 Free PMC article.
References
-
- Als-Nielsen J.; Jacquemain D.; Kjaer K.; Leveiller F.; Lahav M.; Leiserowitz L. Principles and applications of grazing incidence X-ray and neutron scattering from ordered molecular monolayers at the air–water interface. Phys. Rep. 1994, 246, 251–313. 10.1016/0370-1573(94)90046-9. - DOI
-
- Kaganer V. M.; Möhwald H.; Dutta P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. 10.1103/RevModPhys.71.779. - DOI
LinkOut - more resources
Full Text Sources

