Real-Time Observation of CaCO3 Mineralization in Highly Supersaturated Graphene Liquid Cells
- PMID: 32596599
- PMCID: PMC7315570
- DOI: 10.1021/acsomega.0c01300
Real-Time Observation of CaCO3 Mineralization in Highly Supersaturated Graphene Liquid Cells
Abstract
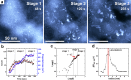
The mineralization dynamics of calcium carbonate is investigated under highly supersaturated conditions using graphene liquid cell transmission electron microscopy. We demonstrate that the mineralization process has three steps: nucleation, diffusion-limited growth, and Ostwald ripening/coalescence. In addition, we show that the polymorphs precipitate in a specific order, from metastable aragonite to stable calcite, thus proving Ostwald's rule of stages. In highly supersaturated solutions, the aragonite phase crystallizes in a stable manner, in addition to the calcite phase.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Blatt H.; Tracy R.; Owens B.. Petrology: Igneous, Sedimentary, and Metamorphic, 3rd ed.; W. H. Freeman & Company: New York, 2006.
-
- Bathurst R. G. C.Carbonate Sediments and Their Diagenesis, 1st ed.; Elsevier: New York, 1972.
-
- Hill C.; Forti P.. Cave Minerals of the World, 2nd ed.; National Speleological Society: Huntsville, Albama, 1997; pp 285–287.
-
- Railsback L. B.; Brook G. A.; Chen J.; Kalin R.; Fleisher C. J. Environmental Controls on the Petrology of a Late Holocene Speleothem from Botswana with Annual Layers of Aragonite and Calcite. J. Sediment. Res., Sect. A 1994, 64, 147–155. 10.1306/d4267d39-2b26-11d7-8648000102c1865d. - DOI
-
- Nancollas G. H.; Reddy M. M. Kinetics of Crystallization of Scale-Forming Minerals. Soc. Pet. Eng. J. 1974, 14, 117–126. 10.2118/4360-pa. - DOI
LinkOut - more resources
Full Text Sources

