New Insights into the Fundamental Principle of Semiconductor Photocatalysis
- PMID: 32596623
- PMCID: PMC7315598
- DOI: 10.1021/acsomega.0c02145
New Insights into the Fundamental Principle of Semiconductor Photocatalysis
Abstract
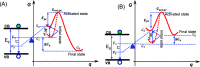
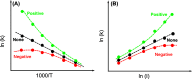
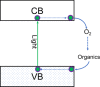
Although photocatalysis has been studied for many years as an attractive way to resolve energy and environmental problems, its principle still remains unclear. Some confusions and misunderstandings exist in photocatalytic studies. This research aims to elaborate some new thoughts on the fundamental principle of semiconductor photocatalysis. Starting from the basic laws of thermodynamics, we first defined the thermodynamic potential of photocatalysis. A concept, the Gibbs potential landscape, was thus then proposed to describe the kinetics of photocatalysis. Photocatalysis is therefore defined as a light-driven chemical reaction that still needs heat activation, in that light and heat play their different roles and interact with each other. Photocatalysis should feature an activation energy functioning with both light and heat. The roles of light and heat are correlative and mutually inhibit at both levels of thermodynamics and kinetics, so it is impossible for an intrinsic light-heat synergism to happen. Two criteria were further proposed to determine an intrinsic light-heat synergism in photocatalysis. Experiments were also carried out to calculate the thermodynamic potential and can agree well with the theory. Experimental results proved that there is no intrinsic light-heat synergism, in accordance with our theoretical prediction. This research clarified some misunderstandings and gained some new insights into the nature of photocatalysis; this is important for the discipline of semiconductor photocatalysis.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Liu B.; Zhao X.; Yu J.; Parkin I. P.; Fujishima A.; Nakata K. Intrinsic Intermediate Gap States of TiO2 Materials and Their Roles in Charge Carrier Kinetics. J. Photochem. Photobio. C: Photochem. Rev. 2019, 39, 1–57. 10.1016/j.jphotochemrev.2019.02.001. - DOI
-
- Fujishima A.; Zhang X.; Tryk D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. 10.1016/j.surfrep.2008.10.001. - DOI
-
- Sun X.; Huang H.; Zhao Q.; Ma T.; Wang L. Thin-Layered Photocatalysts. Adv. Funct. Mater. 2020, 30, 1910005.10.1002/adfm.201910005. - DOI
-
- Lin S.; Zhang Y.; You Y.; Zeng X.; Ma T.; Huang H. Bifunctional Hydrogen Production and Storage on 0D–1D Heterojunction of Cd0.5Zn0.5S@Halloysites. Adv. Funct. Mater. 2019, 29, 1903825.10.1002/adfm.201903825. - DOI
LinkOut - more resources
Full Text Sources

