Iron overload causes a mild and transient increase in acute lung injury
- PMID: 32596989
- PMCID: PMC7322498
- DOI: 10.14814/phy2.14470
Iron overload causes a mild and transient increase in acute lung injury
Abstract
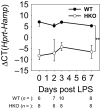
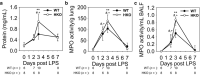
Recent studies have demonstrated a strong link between acute respiratory distress syndrome (ARDS) and the levels of iron and iron-related proteins in the lungs. However, the role of iron overload in ARDS development has yet to be characterized. In this study, we compared the highly iron-overloaded hepcidin knockout mice (HKO) to their iron-sufficient wild-type (WT) littermates in a model of sterile acute lung injury (ALI) induced by treatment with oropharyngeal (OP) LPS. There were no major differences in systemic inflammatory response or airway neutrophil infiltration between the two groups at the time of maximal injury (days 2 and 3) or during the recovery phase (day 7). Hepcidin knockout mice had transiently increased bronchoalveolar lavage fluid (BALF) protein and MPO activity in the lung and BALF on day 3, indicating worse vascular leakage and increased neutrophil activity, respectively. The increased ALI severity in iron-overloaded mice may be a result of increased apoptosis of lung tissue, as evidenced by an increase in cleaved capsase-3 protein in lung homogenates from HKO mice versus WT mice on day 3. Altogether, our data suggest that even severe iron overload has a relatively minor and transient effect in LPS-induced ALI.
Keywords: ARDS; acute lung injury; inflammation; iron overload.
© 2020 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
T.G. and E.N. are shareholders and scientific advisors of Intrinsic LifeSciences and Silarus Therapeutics, and consultants for Ionis Pharmaceuticals, Protagonist, Keryx Pharmaceuticals, La Jolla Pharma, Vifor, Akebia (T.G.), and Gilead (T.G.). Neither A.K. nor V.Z. have any conflicts of interest, financial, or otherwise, to disclose.
Figures



References
-
- Bellani, G. , Laffey, J. G. , Pham, T. , Fan, E. , Brochard, L. , Esteban, A. , … Pesenti, A. ; Investigators LS and Group ET . (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA, 315, 788–800. 10.1001/jama.2016.0291 - DOI - PubMed
-
- Brower, R. G. , Matthay, M. A. , Morris, A. , Schoenfeld, D. , Thompson, B. T. , & Wheeler, A. ; Acute Respiratory Distress Syndrome Network Investigators . (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine, 342, 1301–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

