Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019
- PMID: 32597466
- PMCID: PMC7337637
- DOI: 10.1093/cid/ciaa879
Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019
Abstract
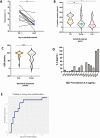
Hyperinflammation is associated with increased mortality in coronavirus disease 2019 (COVID-19). In this retrospective, uncontrolled patient cohort with moderate -severe COVID-19, treatment with baricitinib plus hydroxychloroquine was associated with recovery in 11 of 15 patients. Baricitinib for the treatment of COVID-19 should be further investigated in randomized, controlled clinical trials.
Keywords: COVID-19; baricitinib; hyper-inflammation.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures
Comment in
-
Reply to Jorgensen, et al.Clin Infect Dis. 2021 Dec 6;73(11):e3978-e3979. doi: 10.1093/cid/ciaa1212. Clin Infect Dis. 2021. PMID: 32797235 Free PMC article. No abstract available.
-
Baricitinib: Impact on Coronavirus Disease 2019 (COVID-19) Coagulopathy?Clin Infect Dis. 2021 Dec 6;73(11):e3978-e3979. doi: 10.1093/cid/ciaa1208. Clin Infect Dis. 2021. PMID: 32797237 Free PMC article. No abstract available.
-
Baricitinib as Treatment for Coronavirus Disease 2019 (COVID-19): Friend or Foe of the Pancreas?Clin Infect Dis. 2021 Dec 6;73(11):e3977-e3978. doi: 10.1093/cid/ciaa1209. Clin Infect Dis. 2021. PMID: 32797239 Free PMC article. No abstract available.
References
-
- World Health Organization. Coronavirus disease 2019—situation report number 148. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 17 June 2020.

