Randomized trial of an intensified, multifactorial intervention in patients with advanced-stage diabetic kidney disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan)
- PMID: 32597548
- PMCID: PMC7858124
- DOI: 10.1111/jdi.13339
Randomized trial of an intensified, multifactorial intervention in patients with advanced-stage diabetic kidney disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan)
Abstract
Aims/introduction: We evaluated the efficacy of multifactorial intensive treatment (IT) on renal outcomes in patients with type 2 diabetes and advanced-stage diabetic kidney disease (DKD).
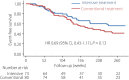
Materials and methods: The Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan) is a multicenter, open-label, randomized controlled trial with a 5-year follow-up period. We randomly assigned 164 patients with advanced-stage diabetic kidney disease (urinary albumin-to-creatinine ratio ≥300 mg/g creatinine, serum creatinine level 1.2-2.5 mg/dL in men and 1.0-2.5 mg/dL in women) to receive either IT or conventional treatment. The primary composite outcome was end-stage kidney failure, doubling of serum creatinine or death from any cause, which was assessed in the intention-to-treat population.
Results: The IT tended to reduce the risk of primary end-points as compared with conventional treatment, but the difference between treatment groups did not reach the statistically significant level (hazard ratio 0.69, 95% confidence interval 0.43-1.11; P = 0.13). Meanwhile, the decrease in serum low-density lipoprotein cholesterol level and the use of statin were significantly associated with the decrease in primary outcome (hazard ratio 1.14; 95% confidence interval 1.05-1.23, P < 0.001 and hazard ratio 0.53, 95% confidence interval 0.28-0.998, P < 0.05, respectively). The incidence of adverse events was not different between treatment groups.
Conclusions: The risk of kidney events tended to decrease by IT, although it was not statistically significant. Lipid control using statin was associated with a lower risk of adverse kidney events. Further follow-up study might show the effect of IT in patients with advanced diabetic kidney disease.
Keywords: Diabetic Nephropathy Remission and Regression Team Trial in Japan; Diabetic kidney disease; Diabetic nephropathy.
© 2020 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
KS received speaker fees from MSD, Eli Lilly Japan, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Mitsubishi Tanabe and Kyowa Hakko Kirin; research support from Takeda, MSD, Kyowa Hakko Kirin and Mitsubishi Tanabe; and a consulting fee from Daiichi Sankyo. MH has received speaker fees from Astellas, Taisho‐Toyama, Tanabe‐Mitsubishi, Boehringer Ingelheim, Taisho, Kowa, Ono, MSD, Novartis and Novo‐Nordisk; and research support from Novo‐Nordisk, Ono, Shionogi and Johnson & Johnson. DK received speaker fees from MSD, Astellas Pharma Inc., Ono, Taisho, Mitsubishi Tanabe, Eli Lilly Japan, Boehringer‐Ingelheim Japan and Novo Nordisk Pharma; and research support from Ono., Taisho, Mitsubishi Tanabe and Boehringer‐Ingelheim Japan. HA received a research grant from Ono and Boehringer Ingelheim GmbH. MN received speaker fees from Mitsubishi Tanabe, Takeda, Kyowa Kirin, Taisho, Novo Nordisk Pharma and Daiichi Sankyo. HM is a consultant for AbbVie, Teijin and Boehringer‐Ingelheim Japan. TN, YS, SM, DS, TY, TU, DO and SM declare no conflict of interest.
Figures


References
-
- Collins AJ, Foley RN, Chavers B, et al US renal data system 2013 annual data report. Am J Kidney Dis 2014; 63: A7. - PubMed
-
- Gerstein H, Mann J, Yi Q, et al Albuminuria and risk of CV events, death, and heart failure in diabetic and non‐diabetic individuals. JAMA 2001; 286: 421–446. - PubMed
-
- Go AS, Chertow GM, Fan D, et al Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1295. - PubMed
-
- Andresdottir G, Jensen ML, Carstensen B, et al Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care 2014; 37: 1660–1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

