Aromatic interactions with membrane modulate human BK channel activation
- PMID: 32597752
- PMCID: PMC7371421
- DOI: 10.7554/eLife.55571
Aromatic interactions with membrane modulate human BK channel activation
Abstract
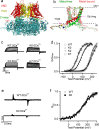
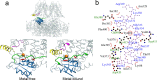
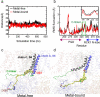
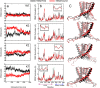
Large-conductance potassium (BK) channels are transmembrane (TM) proteins that can be synergistically and independently activated by membrane voltage and intracellular Ca2+. The only covalent connection between the cytosolic Ca2+ sensing domain and the TM pore and voltage sensing domains is a 15-residue 'C-linker'. To determine the linker's role in human BK activation, we designed a series of linker sequence scrambling mutants to suppress potential complex interplay of specific interactions with the rest of the protein. The results revealed a surprising sensitivity of BK activation to the linker sequence. Combining atomistic simulations and further mutagenesis experiments, we demonstrated that nonspecific interactions of the linker with membrane alone could directly modulate BK activation. The C-linker thus plays more direct roles in mediating allosteric coupling between BK domains than previously assumed. Our results suggest that covalent linkers could directly modulate TM protein function and should be considered an integral component of the sensing apparatus.
Keywords: allosteric coupling; atomistic simulation; channel gating; hydrophobic dewetting; membrane anchoring; molecular biophysics; structural biology; xenopus.
© 2020, Yazdani et al.
Conflict of interest statement
MY, GZ, ZJ, JS, JC, JC No competing interests declared
Figures






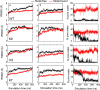

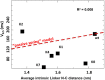


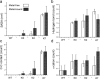





References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Åqvist J, Wennerström P, Nervall M, Bjelic S, Brandsdal BO. Molecular dynamics simulations of water and biomolecules with a monte carlo constant pressure algorithm. Chemical Physics Letters. 2004;384:288–294. doi: 10.1016/j.cplett.2003.12.039. - DOI
-
- Bavro VN, De Zorzi R, Schmidt MR, Muniz JR, Zubcevic L, Sansom MS, Vénien-Bryan C, Tucker SJ. Structure of a KirBac potassium channel with an open bundle crossing indicates a mechanism of channel gating. Nature Structural & Molecular Biology. 2012;19:158–163. doi: 10.1038/nsmb.2208. - DOI - PMC - PubMed
-
- Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M. CHARMM: the biomolecular simulation program. Journal of Computational Chemistry. 2009;30:1545–1614. doi: 10.1002/jcc.21287. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

