Golgi compartments enable controlled biomolecular assembly using promiscuous enzymes
- PMID: 32597757
- PMCID: PMC7360365
- DOI: 10.7554/eLife.49573
Golgi compartments enable controlled biomolecular assembly using promiscuous enzymes
Abstract
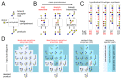
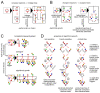
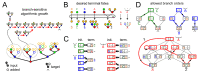
The synthesis of eukaryotic glycans - branched sugar oligomers attached to cell-surface proteins and lipids - is organized like a factory assembly line. Specific enzymes within successive compartments of the Golgi apparatus determine where new monomer building blocks are linked to the growing oligomer. These enzymes act promiscuously and stochastically, causing microheterogeneity (molecule-to-molecule variability) in the final oligomer products. However, this variability is tightly controlled: a given eukaryotic protein type is typically associated with a narrow, specific glycan oligomer profile. Here, we use ideas from the mathematical theory of self-assembly to enumerate the enzymatic causes of oligomer variability and show how to eliminate each cause. We rigorously demonstrate that cells can specifically synthesize a larger repertoire of glycan oligomers by partitioning promiscuous enzymes across multiple Golgi compartments. This places limits on biomolecular assembly: glycan microheterogeneity becomes unavoidable when the number of compartments is limited, or enzymes are excessively promiscuous.
Keywords: cell biology; enzyme promiscuity; glycan synthesis; none; physics of living systems; self-assembly; stochastic chemical reactions.
© 2020, Jaiman and Thattai.
Conflict of interest statement
AJ, MT No competing interests declared
Figures


Similar articles
-
Promiscuity and specificity of eukaryotic glycosyltransferases.Biochem Soc Trans. 2020 Jun 30;48(3):891-900. doi: 10.1042/BST20190651. Biochem Soc Trans. 2020. PMID: 32539082 Free PMC article. Review.
-
Glycan processing in the Golgi as optimal information coding that constrains cisternal number and enzyme specificity.Elife. 2022 Feb 17;11:e76757. doi: 10.7554/eLife.76757. Elife. 2022. PMID: 35175197 Free PMC article.
-
Systems analysis of N-glycan processing in mammalian cells.PLoS One. 2007 Aug 8;2(8):e713. doi: 10.1371/journal.pone.0000713. PLoS One. 2007. PMID: 17684559 Free PMC article.
-
The roles of enzyme localisation and complex formation in glycan assembly within the Golgi apparatus.Curr Opin Cell Biol. 2004 Aug;16(4):356-63. doi: 10.1016/j.ceb.2004.06.007. Curr Opin Cell Biol. 2004. PMID: 15261667 Review.
-
What can yeast tell us about N-linked glycosylation in the Golgi apparatus?FEBS Lett. 2001 Jun 8;498(2-3):223-7. doi: 10.1016/s0014-5793(01)02488-7. FEBS Lett. 2001. PMID: 11412862 Review.
Cited by
-
Congenital disorder of glycosylation caused by starting site-specific variant in syntaxin-5.Nat Commun. 2021 Oct 28;12(1):6227. doi: 10.1038/s41467-021-26534-y. Nat Commun. 2021. PMID: 34711829 Free PMC article.
-
Limits on the computational expressivity of non-equilibrium biophysical processes.Nat Commun. 2025 Aug 5;16(1):7184. doi: 10.1038/s41467-025-61873-0. Nat Commun. 2025. PMID: 40764290 Free PMC article.
-
The stem region of group A transferase is crucial for its specificity, and its alteration promotes heterologous Forssman synthase activity.Sci Rep. 2023 Aug 26;13(1):13996. doi: 10.1038/s41598-023-40900-4. Sci Rep. 2023. PMID: 37634031 Free PMC article.
-
Supply chain logistics - the role of the Golgi complex in extracellular matrix production and maintenance.J Cell Sci. 2022 Jan 1;135(1):jcs258879. doi: 10.1242/jcs.258879. Epub 2022 Jan 13. J Cell Sci. 2022. PMID: 35023559 Free PMC article. Review.
-
GRASP55 regulates intra-Golgi localization of glycosylation enzymes to control glycosphingolipid biosynthesis.EMBO J. 2021 Oct 18;40(20):e107766. doi: 10.15252/embj.2021107766. Epub 2021 Sep 13. EMBO J. 2021. PMID: 34516001 Free PMC article.
References
-
- Adibekian A, Stallforth P, Hecht M-L, Werz DB, Gagneux P, Seeberger PH. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem. Sci. 2011;2:337–344. doi: 10.1039/C0SC00322K. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

