Dynamical modeling of pro- and anti-inflammatory cytokines in the early stage of septic shock
- PMID: 32597796
- PMCID: PMC7505012
- DOI: 10.3233/ISB-200474
Dynamical modeling of pro- and anti-inflammatory cytokines in the early stage of septic shock
Abstract
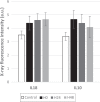
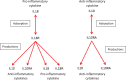
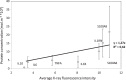
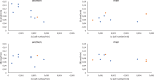
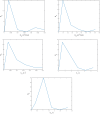
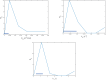
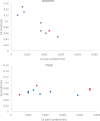
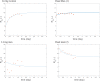
A dynamical model of the pathophysiological behaviors of IL18 and IL10 cytokines with their receptors is tested against data for the case of early sepsis. The proposed approach considers the surroundings (organs and bone marrow) and the different subsystems (cells and cyctokines). The interactions between blood cells, cytokines and the surroundings are described via mass balances. Cytokines are adsorbed onto associated receptors at the cell surface. The adsorption is described by the Langmuir model and gives rise to the production of more cytokines and associated receptors inside the cell. The quantities of pro and anti-inflammatory cytokines present in the body are combined to give global information via an inflammation level function which describes the patient's state. Data for parameter estimation comes from the Sepsis 48 H database. Comparisons between patient data and simulations are presented and are in good agreement. For the IL18/IL10 cytokine pair, 5 key parameters have been found. They are linked to pro-inflammatory IL18 cytokine and show that the early sepsis is driven by components of inflammatory character.
Keywords: Early sepsis; IL18 and IL10 cytokines; dynamic modeling; inflammation; parameter estimation.
Figures







References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.-L. and Angus D.C., The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA 315(8) (2016), 801. - PMC - PubMed
-
- Sakr Y., Jaschinski U., Wittebole X., Szakmany T., Lipman J., Ñamendys-Silva S.A., Martin-Loeches I., Leone M., Lupu M.-N., Vincent J.-L., and ICON Investigators, Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit, Open Forum Infectious Diseases 5(12) (2018). - PMC - PubMed
-
- Reinhart K., Daniels R., Kissoon N., Machado F.R., Schachter R.D. and Finfer S., Recognizing Sepsis as a Global Health Priority — A WHO Resolution, New England Journal of Medicine 377(5) (2017), 414–417. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

