Kv1.3 modulates neuroinflammation and neurodegeneration in Parkinson's disease
- PMID: 32597830
- PMCID: PMC7410064
- DOI: 10.1172/JCI136174
Kv1.3 modulates neuroinflammation and neurodegeneration in Parkinson's disease
Abstract
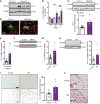
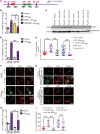
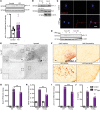
Characterization of the key cellular targets contributing to sustained microglial activation in neurodegenerative diseases, including Parkinson's disease (PD), and optimal modulation of these targets can provide potential treatments to halt disease progression. Here, we demonstrated that microglial Kv1.3, a voltage-gated potassium channel, was transcriptionally upregulated in response to aggregated α-synuclein (αSynAgg) stimulation in primary microglial cultures and animal models of PD, as well as in postmortem human PD brains. Patch-clamp electrophysiological studies confirmed that the observed Kv1.3 upregulation translated to increased Kv1.3 channel activity. The kinase Fyn, a risk factor for PD, modulated transcriptional upregulation and posttranslational modification of microglial Kv1.3. Multiple state-of-the-art analyses, including Duolink proximity ligation assay imaging, revealed that Fyn directly bound to Kv1.3 and posttranslationally modified its channel activity. Furthermore, we demonstrated the functional relevance of Kv1.3 in augmenting the neuroinflammatory response by using Kv1.3-KO primary microglia and the Kv1.3-specific small-molecule inhibitor PAP-1, thus highlighting the importance of Kv1.3 in neuroinflammation. Administration of PAP-1 significantly inhibited neurodegeneration and neuroinflammation in multiple animal models of PD. Collectively, our results imply that Fyn-dependent regulation of Kv1.3 channels plays an obligatory role in accentuating the neuroinflammatory response in PD and identify Kv1.3 as a potential therapeutic target for PD.
Keywords: Inflammation; Neurodegeneration; Neuroscience; Parkinson’s disease; Potassium channels.
Conflict of interest statement
Figures





References
-
- Wolters ECh Non-motor extranigral signs and symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 Suppl 3:S6–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

