Evidence for Expanding Invasive Mediastinal Staging for Peripheral T1 Lung Tumors
- PMID: 32599066
- PMCID: PMC8173766
- DOI: 10.1016/j.chest.2020.05.607
Evidence for Expanding Invasive Mediastinal Staging for Peripheral T1 Lung Tumors
Abstract
Background: Guidelines recommend invasive mediastinal staging for patients with non-small cell lung cancer and a "central" tumor. However, there is no consensus definition for central location. As such, the decision to perform invasive staging largely remains on an empirical foundation.
Research question: Should patients with peripheral T1 lung tumors undergo invasive mediastinal staging?
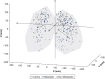
Study design and methods: All participants with a screen-detected cancer with a solid component between 8 and 30 mm were identified from the National Lung Screening Trial. After translation of CT data, cancer location was identified and the X, Y, Z coordinates were determined as well as distance from the main carina. A multivariable logistic regression model was constructed to evaluate for predictors associated with lymph node metastasis.
Results: Three hundred thirty-two participants were identified, of which 69 had lymph node involvement (20.8%). Of those with lymph node metastasis, 39.1% were N2. There was no difference in rate of lymph node metastasis based on tumor size (OR, 1.03; P = .248). There was also no statistical difference in rate of lymph node metastasis based on location, either by distance from the carina (OR, 0.99; P = .156) or tumor coordinates (X: P = .180; Y: P = .311; Z: P = .292). When adjusted for age, sex, histology, and smoking history, there was no change in the magnitude of the risk, and tests of significance were not altered.
Interpretation: Our data indicate a high rate of N2 metastasis among T1 tumors and no significant relationship between tumor diameter or location. This suggests that patients with small, peripheral lung cancers may benefit from invasive mediastinal staging.
Keywords: endobronchial ultrasound-guided transbronchial needle aspiration; lung cancer; staging.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Should We Expand Invasive Mediastinal Staging to Peripheral T1 Lung Tumors?Chest. 2021 Jan;159(1):446-447. doi: 10.1016/j.chest.2020.07.092. Chest. 2021. PMID: 33422218 No abstract available.
-
Response.Chest. 2021 Jan;159(1):447-448. doi: 10.1016/j.chest.2020.08.2078. Chest. 2021. PMID: 33422219 No abstract available.
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer. In: Diagnosis and Management of Lung Cancer, 3rd ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(5 Suppl):e211S-e250S. - PubMed
-
- Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and Management of Lung Cancer, 3rd ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(5 Suppl):7S-37S. - PubMed
-
- De Leyn P., Dooms C., Kuzdzal J. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(5):787–798. - PubMed
-
- National Comprehensive Care Network Non-small cell lung cancer (Version 4.2019) https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed August 20, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials