N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic
- PMID: 32599102
- PMCID: PMC7319649
- DOI: 10.1016/j.ajic.2020.06.194
N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic
Abstract
Background: Response to the COVID-19 pandemic by hospital systems has been strained by severe shortages in personal protective equipment (PPE), particularly N95 respirators. Recently, the Centers for Disease Control and Prevention endorsed decontamination strategies to prolong the lifespan of single use respirators. Battelle and Duke University have validated hospital protocols to decontaminate respirators using vaporized hydrogen peroxide (VHP) at 30%-35% concentrations. To prolong our supply of respirators, we evaluated and implemented VHP decontamination at 59% hydrogen peroxide concentration while detailing the effects of this process on the filtration efficiency and quantitative fit of single-use respirators. This study may help other health systems develop local solutions to their N95 mask shortage during this COVID-19 pandemic.
Methods: N95 respirators (3M 8211 FF and 9210 FF) that were treated with 5 and 10 cycles of VHP by the V-PRO maX Low Temperature Sterilization System were evaluated quantitatively for filtration efficiency as well as with quantitative fit testing per Occupational Safety and Health Administration standards. A decontamination protocol was concurrently implemented at our institution. This process involved depositing used masks, reprocessing, and re-distributing treated masks efficiently back to frontline providers. Furthermore, we implemented patient safety officers on COVID-19/person under investigation units to ensure optimized donning/doffing of respirators through frontline provider education.
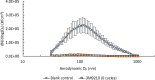
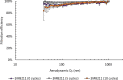
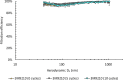
Results: There were no statistically significant changes in mean filtration efficiency between the control and VHP-treated respirators. Furthermore, both treated and untreated respirators demonstrated fit factors above the minimum pass requirement.
Conclusions: We have successfully demonstrated that N95 respirator decontamination with VHP at 59% hydrogen peroxide can be safely utilized to decontaminate single-use N95 respirators without significant effects on filtration efficiency or quantitative fit testing. With the COVID-19 pandemic and N95 respirator shortage, health systems without access to commercial decontamination processes should investigate the viability of such a process in their facilities.
Keywords: Decontamination; N95 filtering facemask.
Copyright © 2020 Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Aerosolized Hydrogen Peroxide Decontamination of N95 Respirators, with Fit-Testing and Viral Inactivation, Demonstrates Feasibility for Reuse during the COVID-19 Pandemic.mSphere. 2022 Oct 26;7(5):e0030322. doi: 10.1128/msphere.00303-22. Epub 2022 Aug 30. mSphere. 2022. PMID: 36040048 Free PMC article.
-
Use, re-use or discard? Quantitatively defined variance in the functional integrity of N95 respirators following vaporized hydrogen peroxide decontamination during the COVID-19 pandemic.J Hosp Infect. 2021 Jan;107:50-56. doi: 10.1016/j.jhin.2020.10.007. Epub 2020 Oct 16. J Hosp Infect. 2021. PMID: 33075406 Free PMC article.
-
Decontaminating N95 respirators during the COVID-19 pandemic: simple and practical approaches to increase decontamination capacity, speed, safety and ease of use.J Hosp Infect. 2021 Mar;109:52-57. doi: 10.1016/j.jhin.2020.12.006. Epub 2020 Dec 19. J Hosp Infect. 2021. PMID: 33347939 Free PMC article.
-
Filtering Facepiece Respirator (N95 Respirator) Reprocessing: A Systematic Review.JAMA. 2021 Apr 6;325(13):1296-1317. doi: 10.1001/jama.2021.2531. JAMA. 2021. PMID: 33656543
-
Decontamination Methods for Reuse of Filtering Facepiece Respirators.JAMA Otolaryngol Head Neck Surg. 2020 Aug 1;146(8):734-740. doi: 10.1001/jamaoto.2020.1423. JAMA Otolaryngol Head Neck Surg. 2020. PMID: 32614377 Free PMC article. Review.
Cited by
-
Comparative evaluation of four hydrogen peroxide-based systems to decontaminate N95 respirators.Antimicrob Steward Healthc Epidemiol. 2021 Aug 18;1(1):e21. doi: 10.1017/ash.2021.183. eCollection 2021. Antimicrob Steward Healthc Epidemiol. 2021. PMID: 36168470 Free PMC article.
-
Plasma-generated reactive water mist for disinfection of N95 respirators laden with MS2 and T4 bacteriophage viruses.Sci Rep. 2022 Nov 19;12(1):19944. doi: 10.1038/s41598-022-23660-5. Sci Rep. 2022. PMID: 36402800 Free PMC article.
-
Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review.Polymers (Basel). 2021 Jun 18;13(12):1998. doi: 10.3390/polym13121998. Polymers (Basel). 2021. PMID: 34207184 Free PMC article. Review.
-
Plastics in the time of COVID-19 pandemic: Protector or polluter?Sci Total Environ. 2021 Mar 10;759:144274. doi: 10.1016/j.scitotenv.2020.144274. Epub 2020 Dec 10. Sci Total Environ. 2021. PMID: 33333331 Free PMC article. Review.
-
Decontamination of N95 respirators against SARS-CoV-2: A scoping review.J Dent. 2021 Jan;104:103534. doi: 10.1016/j.jdent.2020.103534. Epub 2020 Nov 13. J Dent. 2021. PMID: 33197526 Free PMC article.
References
-
- World Health Organization (WHO) Who; 2020. Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19) pp. 1–7. 2019(February)
-
- Ranney ML, Griffeth V, Jha AK. Critical supply shortages — the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. - PubMed
-
- Centers for Disease Control and Prevention. (2020a). COVID-19 Decontamination and Reuse of Filtering Facepiece Respirators.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

