Designing a novel mRNA vaccine against SARS-CoV-2: An immunoinformatics approach
- PMID: 32599237
- PMCID: PMC7319648
- DOI: 10.1016/j.ijbiomac.2020.06.213
Designing a novel mRNA vaccine against SARS-CoV-2: An immunoinformatics approach
Abstract
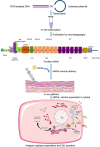
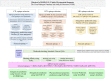
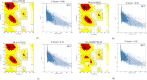
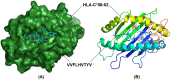
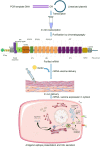
SARS-CoV-2 is the deadly virus behind COVID-19, the disease that went on to ravage the world and caused the biggest pandemic 21st century has witnessed so far. On the face of ongoing death and destruction, the urgent need for the discovery of a vaccine against the virus is paramount. This study resorted to the emerging discipline of immunoinformatics in order to design a multi-epitope mRNA vaccine against the spike glycoprotein of SARS-CoV-2. Various immunoinformatics tools were utilized to predict T and B lymphocyte epitopes. The epitopes were channeled through a filtering pipeline comprised of antigenicity, toxicity, allergenicity, and cytokine inducibility evaluation with the goal of selecting epitopes capable of generating both T and B cell-mediated immune responses. Molecular docking simulation between the epitopes and their corresponding MHC molecules was carried out. 13 epitopes, a highly immunogenic adjuvant, elements for proper sub-cellular trafficking, a secretion booster, and appropriate linkers were combined for constructing the vaccine. The vaccine was found to be antigenic, almost neutral at physiological pH, non-toxic, non-allergenic, capable of generating a robust immune response and had a decent worldwide population coverage. Based on these parameters, this design can be considered a promising choice for a vaccine against SARS-CoV-2.
Keywords: Immunoinformatics; SARS-CoV-2; mRNA vaccine.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures



References
-
- covid19.who.int; WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/?gclid=Cj0KCQjwuJz3BRDTARIsAMg-HxWebhEahKiyYjHOq... [Online]. Available.
-
- www.who.int; Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica... [Online]. Available.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

