Localized mandibular infection affects remote in vivo bioreactor bone generation
- PMID: 32599360
- PMCID: PMC7423761
- DOI: 10.1016/j.biomaterials.2020.120185
Localized mandibular infection affects remote in vivo bioreactor bone generation
Abstract
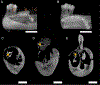
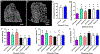
Mandibular reconstruction requires functional and aesthetic repair and is further complicated by contamination from oral and skin flora. Antibiotic-releasing porous space maintainers have been developed for the local release of vancomycin and to promote soft tissue attachment. In this study, mandibular defects in six sheep were inoculated with 106 colony forming units of Staphylococcus aureus; three sheep were implanted with unloaded porous space maintainers and three sheep were implanted with vancomycin-loaded space maintainers within the defect site. During the same surgery, 3D-printed in vivo bioreactors containing autograft or xenograft were implanted adjacent to rib periosteum. After 9 weeks, animals were euthanized, and tissues were analyzed. Antibiotic-loaded space maintainers were able to prevent dehiscence of soft tissue overlying the space maintainer, reduce local inflammatory cells, eliminate the persistence of pathogens, and prevent the increase in mandibular size compared to unloaded space maintainers in this sheep model. Animals with an untreated mandibular infection formed bony tissues with greater density and maturity within the distal bioreactors. Additionally, tissues grown in autograft-filled bioreactors had higher compressive moduli and higher maximum screw pull-out forces than xenograft-filled bioreactors. In summary, we demonstrated that antibiotic-releasing space maintainers are an innovative approach to preserve a robust soft tissue pocket while clearing infection, and that local infections can increase local and remote bone growth.
Keywords: In vivo bioreactors; Large animal model; Local antibiotic release; Mandibular repair; Osteomyelitis; Tissue engineering.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

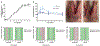



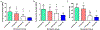
Similar articles
-
Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors.Acta Biomater. 2016 Nov;45:72-84. doi: 10.1016/j.actbio.2016.09.013. Epub 2016 Sep 12. Acta Biomater. 2016. PMID: 27633319
-
Biomaterials-aided mandibular reconstruction using in vivo bioreactors.Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):6954-6963. doi: 10.1073/pnas.1819246116. Epub 2019 Mar 18. Proc Natl Acad Sci U S A. 2019. PMID: 30886100 Free PMC article.
-
Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model.Acta Biomater. 2013 Nov;9(11):8832-9. doi: 10.1016/j.actbio.2013.07.018. Epub 2013 Jul 25. Acta Biomater. 2013. PMID: 23891810
-
Use of porous space maintainers in staged mandibular reconstruction.Oral Maxillofac Surg Clin North Am. 2014 May;26(2):143-9. doi: 10.1016/j.coms.2014.01.002. Oral Maxillofac Surg Clin North Am. 2014. PMID: 24794263 Review.
-
In vivo bioreactors for mandibular reconstruction.J Dent Res. 2014 Dec;93(12):1196-202. doi: 10.1177/0022034514547763. Epub 2014 Aug 19. J Dent Res. 2014. PMID: 25139360 Free PMC article. Review.
Cited by
-
Establishment of a mandible defect model in rabbits infected with multiple bacteria and bioinformatics analysis.Front Bioeng Biotechnol. 2024 Jan 12;12:1350024. doi: 10.3389/fbioe.2024.1350024. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38282893 Free PMC article.
-
Biomaterials-based approaches to mandibular tissue engineering: where we were, where we are, where we are going.Regen Biomater. 2025 Apr 10;12:rbaf024. doi: 10.1093/rb/rbaf024. eCollection 2025. Regen Biomater. 2025. PMID: 40309352 Free PMC article. Review.
-
Choosing the right animal model for osteomyelitis research: Considerations and challenges.J Orthop Translat. 2023 Nov 29;43:47-65. doi: 10.1016/j.jot.2023.10.001. eCollection 2023 Nov. J Orthop Translat. 2023. PMID: 38094261 Free PMC article. Review.
-
[Research progress of in vivo bioreactor for bone tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 May 15;35(5):627-635. doi: 10.7507/1002-1892.202012083. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021. Retraction in: Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 Jan 15;36(1):9. doi: 10.7507/1002-1892.202200001. PMID: 33998218 Free PMC article. Retracted. Review. Chinese.
-
Repair of complex ovine segmental mandibulectomy utilizing customized tissue engineered bony flaps.PLoS One. 2023 Feb 24;18(2):e0280481. doi: 10.1371/journal.pone.0280481. eCollection 2023. PLoS One. 2023. PMID: 36827358 Free PMC article.
References
-
- Shah SR, Tatara AM, Lam J, Lu S, Scott DW, Bennett GN, van den Beucken JJJP, Jansen JA, Wong ME, Mikos AG, Polymer-Based Local Antibiotic Delivery for Prevention of Polymicrobial Infection in Contaminated Mandibular Implants, ACS Biomater. Sci. Eng 2 (2016) 558–566. 10.1021/acsbiomaterials.5b00545. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

