BK Polyomavirus Evades Innate Immune Sensing by Disrupting the Mitochondrial Network and Promotes Mitophagy
- PMID: 32599557
- PMCID: PMC7326741
- DOI: 10.1016/j.isci.2020.101257
BK Polyomavirus Evades Innate Immune Sensing by Disrupting the Mitochondrial Network and Promotes Mitophagy
Abstract
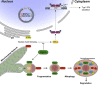
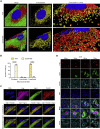
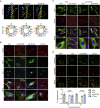
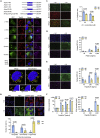
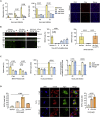
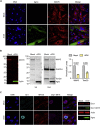
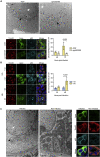
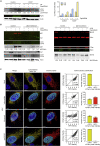
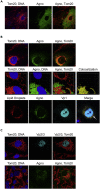
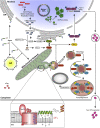
Immune escape contributes to viral persistence, yet little is known about human polyomaviruses. BK-polyomavirus (BKPyV) asymptomatically infects 90% of humans but causes premature allograft failure in kidney transplant patients. Despite virus-specific T cells and neutralizing antibodies, BKPyV persists in kidneys and evades immune control as evidenced by urinary shedding in immunocompetent individuals. Here, we report that BKPyV disrupts the mitochondrial network and membrane potential when expressing the 66aa-long agnoprotein during late replication. Agnoprotein is necessary and sufficient, using its amino-terminal and central domain for mitochondrial targeting and network disruption, respectively. Agnoprotein impairs nuclear IRF3-translocation, interferon-beta expression, and promotes p62/SQSTM1-mitophagy. Agnoprotein-mutant viruses unable to disrupt mitochondria show reduced replication and increased interferon-beta expression but can be rescued by type-I interferon blockade, TBK1-inhibition, or CoCl2-treatment. Mitochondrial fragmentation and p62/SQSTM1-autophagy occur in allograft biopsies of kidney transplant patients with BKPyV nephropathy. JCPyV and SV40 infection similarly disrupt mitochondrial networks, indicating a conserved mechanism facilitating polyomavirus persistence and post-transplant disease.
Keywords: Biological Sciences; Cell Biology; Immunology; Virology.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
Similar articles
-
JC Polyomavirus Infection of Primary Human Renal Epithelial Cells Is Controlled by a Type I IFN-Induced Response.mBio. 2016 Jul 5;7(4):e00903-16. doi: 10.1128/mBio.00903-16. mBio. 2016. PMID: 27381292 Free PMC article.
-
The Presumed Polyomavirus Viroporin VP4 of Simian Virus 40 or Human BK Polyomavirus Is Not Required for Viral Progeny Release.J Virol. 2016 Oct 28;90(22):10398-10413. doi: 10.1128/JVI.01326-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27630227 Free PMC article.
-
BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia.Microb Pathog. 2016 Aug;97:204-8. doi: 10.1016/j.micpath.2016.06.015. Epub 2016 Jun 16. Microb Pathog. 2016. PMID: 27317859
-
[New, newer, newest human polyomaviruses: how far?].Mikrobiyol Bul. 2013 Apr;47(2):362-81. doi: 10.5578/mb.5377. Mikrobiyol Bul. 2013. PMID: 23621738 Review. Turkish.
-
The human polyomavirus BK (BKPyV): virological background and clinical implications.APMIS. 2013 Aug;121(8):728-45. doi: 10.1111/apm.12134. Epub 2013 Jun 19. APMIS. 2013. PMID: 23782063 Review.
Cited by
-
Non-permissive human conventional CD1c+ dendritic cells enable trans-infection of human primary renal tubular epithelial cells and protect BK polyomavirus from neutralization.PLoS Pathog. 2021 Feb 16;17(2):e1009042. doi: 10.1371/journal.ppat.1009042. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33592065 Free PMC article.
-
Unravelling the Immunomodulatory Effects of Viral Ion Channels, towards the Treatment of Disease.Viruses. 2021 Oct 27;13(11):2165. doi: 10.3390/v13112165. Viruses. 2021. PMID: 34834972 Free PMC article. Review.
-
Variations in BK Polyomavirus Immunodominant Large Tumor Antigen-Specific 9mer CD8 T-Cell Epitopes Predict Altered HLA-Presentation and Immune Failure.Viruses. 2020 Dec 21;12(12):1476. doi: 10.3390/v12121476. Viruses. 2020. PMID: 33371492 Free PMC article.
-
Modelling BK Polyomavirus dissemination and cytopathology using polarized human renal tubule epithelial cells.PLoS Pathog. 2023 Aug 28;19(8):e1011622. doi: 10.1371/journal.ppat.1011622. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37639485 Free PMC article.
-
Donor Variability and PD-1 Expression Limit BK Polyomavirus-specific T-cell Function and Therapy.Transplantation. 2025 Sep 1;109(9):1526-1539. doi: 10.1097/TP.0000000000005399. Epub 2025 Apr 9. Transplantation. 2025. PMID: 40200394 Free PMC article.
References
-
- Bernhoff E., Gutteberg T.J., Sandvik K., Hirsch H.H., Rinaldo C.H. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am. J. Transplant. 2008;8:1413–1422. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

