A Capillary-Perfused, Nanocalorimeter Platform for Thermometric Enzyme-Linked Immunosorbent Assay with Femtomole Sensitivity
- PMID: 32599750
- PMCID: PMC7344772
- DOI: 10.3390/bios10060071
A Capillary-Perfused, Nanocalorimeter Platform for Thermometric Enzyme-Linked Immunosorbent Assay with Femtomole Sensitivity
Abstract
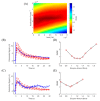
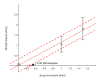
Enzyme-catalyzed chemical reactions produce heat. We developed an enclosed, capillary-perfused nanocalorimeter platform for thermometric enzyme-linked immunosorbent assay (TELISA). We used catalase as enzymes to model the thermal characteristics of the micromachined calorimeter. Model-assisted signal analysis was used to calibrate the nanocalorimeter and to determine reagent diffusion, enzyme kinetics, and enzyme concentration. The model-simulated signal closely followed the experimental signal after selecting for the enzyme turnover rate (kcat) and the inactivation factor (InF), using a known label enzyme amount (Ea). Over four discrete runs (n = 4), the minimized model root mean square error (RMSE) returned 1.80 ± 0.54 fmol for the 1.5 fmol experiments, and 1.04 ± 0.37 fmol for the 1 fmol experiments. Determination of enzyme parameters through calibration is a necessary step to track changing enzyme kinetic characteristics and improves on previous methods to determine label enzyme amounts on the calorimeter platform. The results obtained using model-system signal analysis for calibration led to significantly improved nanocalorimeter platform performance.
Keywords: ELISA; biosensor; microfabricated calorimeter; model-assisted signal analysis; thermometric ELISA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Premjeet S., Deepika G., Sudeep B., Sonam J., Sahil K., Devashish R., Sunil K. Enzyme-Linked Immuno-Sorbent Assay (ELISA), basics and it’s application: A comprehensive review. J. Pharm. Res. 2011;4:4581–4583.
-
- Metkar S.K., Girigoswami K. Diagnostic biosensors in medicine: A review. Biocatal. Agric. Biotech. 2019;17:271–283. doi: 10.1016/j.bcab.2018.11.029. - DOI
-
- Eivazzadeh-Keihan R., Pashazadeh-Panahi P., Mahmoudi T., Chenab K.K., Baradaran B., Hashemzaei M., Radinekiyan F., Mokhtarzadeh A., Maleki A. Dengue virus: A review on advances in detection and trends—from conventional methods to novel biosensors. Microchim. Acta. 2019;186:329. doi: 10.1007/s00604-019-3420-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

