Reversible Oxidative Modifications in Myoglobin and Functional Implications
- PMID: 32599765
- PMCID: PMC7346209
- DOI: 10.3390/antiox9060549
Reversible Oxidative Modifications in Myoglobin and Functional Implications
Abstract
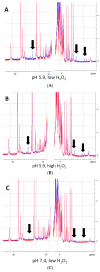
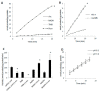
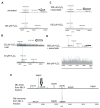
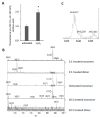
Myoglobin (Mb), an oxygen-binding heme protein highly expressed in heart and skeletal muscle, has been shown to undergo oxidative modifications on both an inter- and intramolecular level when exposed to hydrogen peroxide (H2O2) in vitro. Here, we show that exposure to H2O2 increases the peroxidase activity of Mb. Reaction of Mb with H2O2 causes covalent binding of heme to the Mb protein (Mb-X), corresponding to an increase in peroxidase activity when ascorbic acid is the reducing co-substrate. Treatment of H2O2-reacted Mb with ascorbic acid reverses the Mb-X crosslink. Reaction with H2O2 causes Mb to form dimers, trimers, and larger molecular weight Mb aggregates, and treatment with ascorbic acid regenerates Mb monomers. Reaction of Mb with H2O2 causes formation of dityrosine crosslinks, though the labile nature of the crosslinks broken by treatment with ascorbic acid suggests that the reversible aggregation of Mb is mediated by crosslinks other than dityrosine. Disappearance of a peptide containing a tryptophan residue when Mb is treated with H2O2 and the peptide's reappearance after subsequent treatment with ascorbic acid suggest that tryptophan side chains might participate in the labile crosslinking. Taken together, these data suggest that while exposure to H2O2 causes Mb-X formation, increases Mb peroxidase activity, and causes Mb aggregation, these oxidative modifications are reversible by treatment with ascorbic acid. A caveat is that future studies should demonstrate that these and other in vitro findings regarding properties of Mb have relevance in the intracellular milieu, especially in regard to actual concentrations of metMb, H2O2, and ascorbate that would be found in vivo.
Keywords: ditryptophan; dityrosine; myoglobin; peroxidase; protein aggregation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Myoglobin as a versatile peroxidase: Implications for a more important role for vertebrate striated muscle in antioxidant defense.Comp Biochem Physiol B Biochem Mol Biol. 2019 Aug;234:9-17. doi: 10.1016/j.cbpb.2019.04.005. Epub 2019 Apr 30. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 31051268 Free PMC article.
-
The role of distal histidine in peroxidase activity of myoglobin--transient-kinetics study of the reaction of H2O2 with wild-type and distal-histidine-mutanted recombinant human myoglobin.Eur J Biochem. 1998 Nov 1;257(3):547-55. doi: 10.1046/j.1432-1327.1998.2570547.x. Eur J Biochem. 1998. PMID: 9839942
-
Structures of K42N and K42Y sperm whale myoglobins point to an inhibitory role of distal water in peroxidase activity.Acta Crystallogr D Biol Crystallogr. 2014 Nov;70(Pt 11):2833-9. doi: 10.1107/S1399004714017787. Epub 2014 Oct 16. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 25372675
-
Involvement of the oxygen storage protein myoglobin in muscle damage under oxidative stress.Adv Exp Med Biol. 1998;454:219-23. doi: 10.1007/978-1-4615-4863-8_26. Adv Exp Med Biol. 1998. PMID: 9889895
-
Hemoglobin and Myoglobin as Reducing Agents in Biological Systems. Redox Reactions of Globins with Copper and Iron Salts and Complexes.Biochemistry (Mosc). 2016 Dec;81(13):1735-1753. doi: 10.1134/S0006297916130101. Biochemistry (Mosc). 2016. PMID: 28260494 Review.
Cited by
-
A novel catalytic heme cofactor in SfmD with a single thioether bond and a bis-His ligand set revealed by a de novo crystal structural and spectroscopic study.Chem Sci. 2021 Jan 22;12(11):3984-3998. doi: 10.1039/d0sc06369j. Chem Sci. 2021. PMID: 34163669 Free PMC article.
-
Monitoring the Redox Status in Multiple Sclerosis.Biomedicines. 2020 Oct 12;8(10):406. doi: 10.3390/biomedicines8100406. Biomedicines. 2020. PMID: 33053739 Free PMC article. Review.
-
Hidden Complexity in the Mechanism of the Autoreduction of Myoglobin Compound II.ACS Omega. 2022 Jun 16;7(26):22906-22914. doi: 10.1021/acsomega.2c02798. eCollection 2022 Jul 5. ACS Omega. 2022. PMID: 35811930 Free PMC article.
-
Effects of low-dose sodium nitrite on the structure of yak meat myoglobin during wet curing.Food Chem X. 2022 Aug 23;15:100434. doi: 10.1016/j.fochx.2022.100434. eCollection 2022 Oct 30. Food Chem X. 2022. PMID: 36211786 Free PMC article.
-
Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties.Int J Mol Sci. 2023 Dec 7;24(24):17236. doi: 10.3390/ijms242417236. Int J Mol Sci. 2023. PMID: 38139065 Free PMC article.
References
-
- Kim N.H., Jeong M.S., Choi S.Y., Kang J.H. Oxidative modification of cytochrome c by hydrogen peroxide. Mol. Cells. 2006;22:220–227. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

