Personalized dosing of nicotine replacement therapy versus standard dosing for the treatment of individuals with tobacco dependence: study protocol for a randomized placebo-controlled trial
- PMID: 32600406
- PMCID: PMC7325031
- DOI: 10.1186/s13063-020-04532-7
Personalized dosing of nicotine replacement therapy versus standard dosing for the treatment of individuals with tobacco dependence: study protocol for a randomized placebo-controlled trial
Abstract
Background: Medications for smoking cessation are currently only effective in helping a minority of smokers quit. Drug development is slow and expensive; as such, there is much interest in optimizing the effectiveness of existing treatments and medications. Current standard doses of nicotine replacement therapy are not effective for many smokers, and in many cases, the amount of nicotine provided is much less than when a smoker is smoking their usual number of cigarettes. The proposed study will test if titrating the dose of the nicotine patch (up to 84 mg) will improve quitting success compared to those receiving a 21-mg nicotine patch with increasing doses of placebo patch.
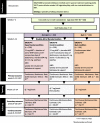
Methods: This is a multicenter, pragmatic, two-arm, placebo-controlled, block randomized controlled trial. We will recruit participants who smoke at least 10 cigarettes daily and are interested in making a quit attempt. After 2 weeks of usual treatment with a 21-mg patch, participants who fail to quit smoking (target n = 400) will be randomized to receive escalating doses of a nicotine patch vs matching placebo patches for an additional 10 weeks or up to a maximum dose of 84 mg per day. Those who stop smoking during the first 2 weeks of usual treatment will continue with 21 mg patch treatment for 10 weeks and will form an additional comparison arm. In addition to the medication, participants will receive brief behavioral counseling at each study visit. The primary outcome will be biochemically confirmed continuous abstinence from smoking during the last 4 weeks of treatment (weeks 9 to 12).
Discussion: Research evidence supporting the effectiveness of personalized doses of nicotine replacement therapy could change current practice in a variety of healthcare settings. Given the evidence that quitting smoking at any age diminishes the risk of tobacco-related morbidity and mortality, even small increases in absolute quit rates can have a substantial population-level impact on reducing smoking-related disease, mortality rates, and associated healthcare costs.
Trial registration: ClinicalTrials.gov, NCT03000387 . Registered on 22 December 2016.
Keywords: Dose titration; Nicotine patches; Nicotine replacement therapy; Placebo patches; Smoking cessation.
Conflict of interest statement
LZ has received funding that includes salary support from Pfizer Inc. (GRAND Award) and the Health Services Research Fund from the Ontario Ministry of Health. PS declares that Johnson & Johnson, Novartis, and Pfizer Inc. are vendors of record for having provided free/discounted smoking cessation pharmacotherapy for other research studies unrelated to this study. RFT has consulted for Ethismos and Quinn Emanuel. BLF has obtained funding from Pfizer Inc. (GRAND Awards, including salary support) for investigator-initiated projects and has received a coil for TMS smoking cessation study from Brainsway. Other funding sources are not related to smoking studies. All other authors declare that they have no competing interests.
Figures

References
-
- Government of Canada . Canadian Tobacco, Alcohol and Drugs Survey (CTADS): summary of results for 2017. 2019.
-
- Jha P. Avoidable deaths from smoking: a global perspective. Public Health Rev. 2011;33(2):569–600. doi: 10.1007/BF03391651. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

