Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway
- PMID: 32600435
- PMCID: PMC7322868
- DOI: 10.1186/s13287-020-01756-x
Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway
Abstract
Background: After surgery, wound recovery in diabetic patients may be disrupted due to delayed inflammation, which can lead to undesired consequences, and there is currently a lack of effective measures to address this issue. Mesenchymal stem cell (MSC)-derived exosomes (Exo) have been proven to be appropriate candidates for diabetic wound healing through the anti-inflammatory effects. In this study, we investigated whether melatonin (MT)-pretreated MSCs-derived exosomes (MT-Exo) could exert superior effects on diabetic wound healing, and we attempted to elucidate the underlying mechanism.
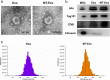
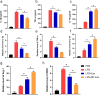
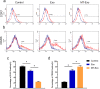
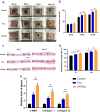
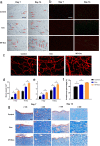
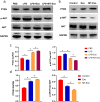
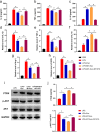
Methods: For the evaluation of the anti-inflammatory effect of MT-Exo, in vitro and in vivo studies were performed. For in vitro research, we detected the secreted levels of inflammation-related factors, such as IL-1β, TNF-α and IL-10 via ELISA and the relative gene expression of the IL-1β, TNF-α, IL-10, Arg-1 and iNOS via qRT-PCR and investigated the expression of PTEN, AKT and p-AKT by Western blotting. For in vivo study, we established air pouch model and streptozotocin (STZ)-treated diabetic wound model, and evaluated the effect of MT-Exo by flow cytometry, optical imaging, H&E staining, Masson trichrome staining, immunohistochemical staining, immunofluorescence, and qRT-PCR (α-SMA, collagen I and III).
Results: MT-Exo significantly suppressed the pro-inflammatory factors IL-1β and TNF-α and reduced the relative gene expression of IL-1β, TNF-α and iNOS, while promoting the anti-inflammatory factor IL-10 along with increasing the relative expression of IL-10 and Arg-1, compared with that of the PBS, LPS and the Exo groups in vitro. This effect was mediated by the increased ratio of M2 polarization to M1 polarization through upregulating the expression of PTEN and inhibiting the phosphorylation of AKT. Similarly, MT-Exo significantly promoted the healing of diabetic wounds by inhibiting inflammation, thereby further facilitating angiogenesis and collagen synthesis in vivo.
Conclusions: MT-Exo could promote diabetic wound healing by suppressing the inflammatory response, which was achieved by increasing the ratio of M2 polarization to M1 polarization through activating the PTEN/AKT signalling pathway, and the pretreatment of MT was proved to be a promising method for treating diabetic wound healing.
Keywords: Diabetic wound; Exosome; Inflammation; Macrophage polarization; Melatonin; Mesenchymal stem cell.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mehta SK, Breitbart EA, Berberian WS, Liporace FA, Lin SS. Bone and wound healing in the diabetic patient. Foot Ankle Clin. 2010;15(3):411–437. - PubMed
-
- Chahal J, Stephen DJ, Bulmer B, Daniels T, Kreder HJ. Factors associated with outcome after subtalar arthrodesis. J Orthop Trauma. 2006;20(8):555–561. - PubMed
-
- He R, Yin H, Yuan B, et al. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol Immunol. 2017;90:42–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

