Development of a multivariable improvement measure for gout
- PMID: 32600452
- PMCID: PMC7325077
- DOI: 10.1186/s13075-020-02254-4
Development of a multivariable improvement measure for gout
Abstract
Background: Gout is a heterogeneous inflammatory disease with numerous clinical manifestations. A composite means to assess the impact of therapy on numerous aspects of gout could be useful.
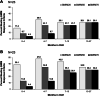
Methods: Results from patients treated with pegloticase or placebo in two randomized clinical trials and their open-label extension were assessed using a novel evidence-based Gout Multivariable Improvement Measure (GMIM) derived from previously reported criteria for remission and complete response. Improvement was defined as serum urate (sU) < 6 mg/dL and absence of flares during the preceding 3 months plus 20, 50, and 70% improvement in tophus size, patient global assessment, pain, and swollen and tender joints.
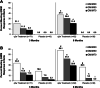
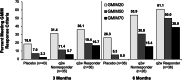
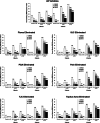
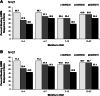
Results: Patients treated with pegloticase manifested a significantly greater GMIM20, 50, and 70 response vs those treated with placebo (GMIM20 at 6 months 37.1% vs 0%, respectively). Higher response rates were significantly more frequent in subjects with persistent urate lowering (GMIM 58.1% at 6 months) in response to pegloticase versus those with only transient urate lowering (GMIM 7.1% at 6 months). However, when the requirement for a decrease in sU to < 6 mg/dL was omitted, a substantial percentage of subjects with transient urate lowering met the GMIM clinical criteria. A sensitivity analysis indicated that gout flares contributed minimally to the model. The response measured by GMIM persisted into the open-level extension for as long as 2 years. Finally, subjects who received placebo in the randomized control trials, but pegloticase in the open-label extension, manifested GMIM responses comparable to that noted with pegloticase-treated subjects in the randomized controlled trials.
Conclusions: GMIM captures changes in disease activity in response to treatment with pegloticase and may serve as an evidence-based tool for assessment of responses to other urate-lowering therapies in gout patients.
Keywords: Disease activity; Gout; Pegloticase.
Conflict of interest statement
NS reports research grants from Pfizer and Amgen and advisory board/consulting fees from Novartis, Horizon Therapeutics, Selecta Biosciences, Olatec, IFM Therapeutics, and Mallinckrodt Pharmaceuticals.
NLE reports consulting fees from Astra Zeneca, Horizon Therapeutics, Ironwood Pharmaceuticals, and SOBI International.
AEY reports consulting from Horizon Therapeutics.
PEL reports consulting fees from Horizon Therapeutics.
Figures

References
-
- Pincus T, Bergman MJ, Maclean R, Yazici Y. Complex measures and indices for clinical research compared with simple patient questionnaires to assess function, pain, and global estimates as rheumatology “vital signs” for usual clinical care. Rheum Dis Clin N Am. 2009;35:779–786. doi: 10.1016/j.rdc.2009.10.010. - DOI - PubMed
-
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. doi: 10.1136/ard.2007.084459. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

