Cutaneous wound healing: canine allogeneic ASC therapy
- PMID: 32600465
- PMCID: PMC7325024
- DOI: 10.1186/s13287-020-01778-5
Cutaneous wound healing: canine allogeneic ASC therapy
Abstract
Background: Wound healing is a complex biological process comprised of a series of sequential events aiming to repair injured tissue. Adult mesenchymal stem cells (MSCs) have been used in cellular therapy in preclinical animal studies; a promising source of MSCs is adipose tissue (AT). In this paper, we evaluated the clinical value and safety of the application of cultured allogenic MSCs from AT for acute and chronic skin wound healing in a canine model.
Methods: Twenty-four dogs of different breeds between 1 and 10 years of age with acute and chronic wounds were studied. Morphology of the wounded skin was monitored for changes over time via serial photographs and histopathological studies.
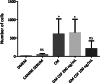
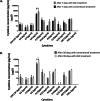
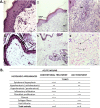
Results: The percentage of the wounds that exhibited contraction and re-epithelialization were significantly different between wounds treated with adipose mesenchymal stem cells (ASCs) and control wounds; this effect was observed in both acute and chronic conditions. At 90 days, re-epithelization of acute and chronic wounds reached more than 97%. Histopathological study revealed a reduction in inflammatory infiltrate and the presence of multiple hair follicles on day 7 after treatment with ASCs, promoting epidermal and dermal regeneration. To guarantee the safety of our treatment, we determined the serum levels of cytokine markers in our patients. ASC treatment upregulated granulocyte-macrophage colony stimulating factor (GM-CSF) at the gene level, which may contribute to the recruitment of cells that participate in skin repair to the site of injury.
Conclusions: The development of an allogenic ASC therapy to improve wound healing in a canine model could have a clinical impact in human treatment.
Keywords: ASC therapy; Adipose mesenchymal stem cells; Canine cutaneous wounds; Regenerative medicine.
Conflict of interest statement
The authors declare they have no competing interests.
Figures



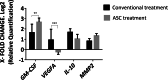
References
-
- Alstrup T, Eijken M, Bohn AB, Møller B, Damsgaard TE. Isolation of adipose tissue-derived stem cells: enzymatic digestion in combination with mechanical distortion to increase adipose tissue-derived stem cell yield from human aspirated fat. Curr Protoc Stem Cell Biol. 2019;48:e68. - PubMed
-
- Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, Garcovich S. Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): enzymatic digestion versus mechanical centrifugation. Int J Mol Sci. 2019;20:5471. - PMC - PubMed
-
- Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1:230–236. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

