Number needed to immunize to prevent RSV with extended half-life monoclonal antibody
- PMID: 32600912
- PMCID: PMC8684408
- DOI: 10.1016/j.vaccine.2020.06.034
Number needed to immunize to prevent RSV with extended half-life monoclonal antibody
Abstract
Background: Respiratory syncytial virus (RSV) is one of the most important respiratory pathogens in young children. Infants <6 months of age and infants and young children with extreme pre-term birth, and cardiac and pulmonary co-morbidities experience the highest incidence of severe RSV disease. There are no licensed vaccines; immunoprophylaxis is recommended for the highest risk children. Extended half-life RSV monoclonal antibodies (EHL-mAbs) are under development intended for immunization of all infants and high-risk children <2 years of age. We modeled the anticipated public health benefits of RSV EHL-mAb immunization using the number needed to immunize (NNI).
Methods: We combined RSV hospitalization, outpatient and outpatient lower respiratory tract infection (LRI) incidence estimates and a range of immunization efficacies to estimate the annual NNI. We calculated the absolute incidence rate reduction (ARR) by multiplying the incidence rates by immunization efficacy. NNI was calculated as the reciprocal of the ARR.
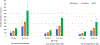
Results: For an RSV EHL-mAb with 70% efficacy, 6-18 infants would need to be immunized to prevent one RSV-associated outpatient visit, and 13-33 infants would need to be immunized to prevent one RSV-associated LRI outpatient visit. To prevent one RSV-associated hospitalization, 37-85 infants 0-5 months of age, and 107-280 infants 6-11 months of age would need to be immunized.
Conclusions: Public health benefits, such as disease cases averted due to immunization, are essential elements in consideration of candidate vaccines for a national immunization program. An RSV EHL-mAb of moderate efficacy could have high impact. These data provide an additional perspective for public health decision making.
Keywords: Immunization; Number needed to vaccinate; RSV monoclonal antibody; mAb.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
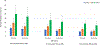
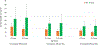
References
-
- Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019;8:284–6. - PubMed
-
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275–84. - PubMed
-
- Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J 2012;31:5–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

