Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis
- PMID: 32601075
- PMCID: PMC8792803
- DOI: 10.1158/1078-0432.CCR-20-0953
Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis
Abstract
Purpose: Uterine-serous-carcinoma (USC) is an aggressive variant of endometrial cancer. On the basis of preliminary results of a multicenter, randomized phase II trial, trastuzumab (T), a humanized-mAb targeting Her2/Neu, in combination with carboplatin/paclitaxel (C/P), is recognized as an alternative in treating advanced/recurrent HER2/Neu-positive USC. We report the updated survival analysis of NCT01367002.
Patients and methods: Eligible patients had stage III to IV or recurrent disease. Participants were randomized 1:1 to receive C/P for six cycles ± T followed by maintenance T until progression or toxicity. Progression-free survival (PFS) was the primary endpoint; overall survival (OS) and toxicity were secondary endpoints.
Results: Sixty-one patients were randomized. After a median-follow-up of 25.9 months, 43 progressions and 38 deaths occurred among 58 evaluable patients. Updated median-PFS continued to favor the T-arm, with medians of 8.0 months versus 12.9 months in the control and T-arms (HR = 0.46; 90% CI, 0.28-0.76; P = 0.005). Median-PFS was 9.3 months versus 17.7 months among 41 patients with stage III to IV disease undergoing primary treatment (HR = 0.44; 90% CI, 0.23-0.83; P = 0.015), and 7.0 months versus 9.2 months among 17 patients with recurrent disease (HR = 0.12; 90% CI, 0.03-0.48; P = 0.004). OS was higher in the T compared with the control arm, with medians of 29.6 months versus 24.4 months (HR = 0.58; 90% CI, 0.34-0.99; P = 0.046). The benefit was most notable in those with stage III to IV disease, with survival median not reached in the T-arm versus 24.4 months in the control arm (HR = 0.49; 90% CI, 0.25-0.97; P = 0.041). Toxicity was not different between arms.
Conclusions: Addition of T to C/P increased PFS and OS in women with advanced/recurrent HER2/Neu-positive USC, with the greatest benefit seen for the treatment of stage III to IV disease.
©2020 American Association for Cancer Research.
Figures



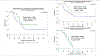
References
-
- Hameed K, Morgan DA. Papillary adenocarcinoma of endometrium with psammoma bodies. Histology and fine structure. Cancer. 1972;29(5):1326–1335. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

