Deep brain stimulation in early-stage Parkinson disease: Five-year outcomes
- PMID: 32601120
- PMCID: PMC7455319
- DOI: 10.1212/WNL.0000000000009946
Deep brain stimulation in early-stage Parkinson disease: Five-year outcomes
Abstract
Objective: To report 5-year outcomes from the subthalamic nucleus (STN) deep brain stimulation (DBS) in early-stage Parkinson disease (PD) pilot clinical trial.
Methods: The pilot was a prospective, single-blind clinical trial that randomized patients with early-stage PD (Hoehn & Yahr II off medications) to receive bilateral STN DBS plus optimal drug therapy (ODT) vs ODT alone (IDEG050016, NCT0282152, IRB040797). Participants who completed the 2-year trial participated in this observational follow-up study, which included annual outpatient visits through 5 years. This analysis includes 28 patients who were taking PD medications for 6 months to 4 years at enrollment. Outcomes were analyzed using both proportional odds logistic regression and linear mixed effects models.
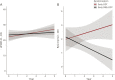
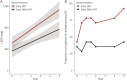
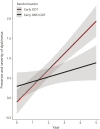
Results: Early STN DBS + ODT participants required lower levodopa equivalent daily doses (p = 0.04, β = -240 mg, 95% confidence interval [CI] -471 to -8) and had 0.06 times the odds of requiring polypharmacy at 5 years compared to early ODT participants (p = 0.01, odds ratio [OR] 0.06, 95% CI 0.00 to 0.65). The odds of having worse rest tremor for early STN DBS + ODT participants were 0.21 times those of early ODT participants (p < 0.001, OR 0.21, 95% CI 0.09 to 0.45). The safety profile was similar between groups.
Conclusions: These results suggest that early DBS reduces the need for and complexity of PD medications while providing long-term motor benefit over standard medical therapy. Further investigation is warranted, and the Food and Drug Administration has approved the conduct of a prospective, multicenter, pivotal clinical trial of DBS in early-stage PD (IDEG050016).
Classification of evidence: This study provides Class II evidence that DBS implanted in early-stage PD decreases the risk of disease progression and polypharmacy compared to optimal medical therapy alone.
Trial registration: ClinicalTrials.gov NCT00282152.
© 2020 American Academy of Neurology.
Figures
Comment in
-
Parkinson disease: The long-term benefits of early use of deep brain stimulation.Neurology. 2020 Jul 28;95(4):e436-e438. doi: 10.1212/WNL.0000000000009952. Neurology. 2020. PMID: 32719067 No abstract available.
-
Reader Response: Deep Brain Stimulation in Early-Stage Parkinson Disease: Five-Year Outcomes.Neurology. 2021 Mar 23;96(12):590-591. doi: 10.1212/WNL.0000000000011641. Neurology. 2021. PMID: 33753526 No abstract available.
-
Author Response: Deep Brain Stimulation in Early-Stage Parkinson Disease: Five-Year Outcomes.Neurology. 2021 Mar 23;96(12):591. doi: 10.1212/WNL.0000000000011651. Neurology. 2021. PMID: 33753527 No abstract available.
-
Reader Response: Deep Brain Stimulation in Early-Stage Parkinson Disease: Five-Year Outcomes.Neurology. 2021 Mar 23;96(12):591-592. doi: 10.1212/WNL.0000000000011648. Neurology. 2021. PMID: 33753528 No abstract available.
-
Author Response: Deep Brain Stimulation in Early-Stage Parkinson Disease: Five-Year Outcomes.Neurology. 2021 Mar 23;96(12):592. doi: 10.1212/WNL.0000000000011650. Neurology. 2021. PMID: 33753529 No abstract available.
References
-
- Finder SG, Bliton MJ, Gill CE, et al. . Potential subjects' responses to an ethics questionnaire in a phase I study of deep brain stimulation in early Parkinson's disease. J Clin Ethics 2012;23:207–216. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
