A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin
- PMID: 32601161
- PMCID: PMC7449190
- DOI: 10.1128/AAC.02487-19
A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin
Abstract
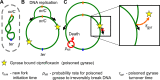
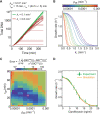
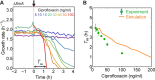
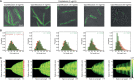
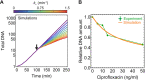
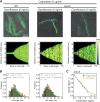
Fluoroquinolones, antibiotics that cause DNA damage by inhibiting DNA topoisomerases, are clinically important, but their mechanism of action is not yet fully understood. In particular, the dynamical response of bacterial cells to fluoroquinolone exposure has hardly been investigated, although the SOS response, triggered by DNA damage, is often thought to play a key role. Here, we investigated the growth inhibition of the bacterium Escherichia coli by the fluoroquinolone ciprofloxacin at low concentrations. We measured the long-term and short-term dynamical response of the growth rate and DNA production rate to ciprofloxacin at both the population and single-cell levels. We show that, despite the molecular complexity of DNA metabolism, a simple roadblock-and-kill model focusing on replication fork blockage and DNA damage by ciprofloxacin-poisoned DNA topoisomerase II (gyrase) quantitatively reproduces long-term growth rates in the presence of ciprofloxacin. The model also predicts dynamical changes in the DNA production rate in wild-type E. coli and in a recombination-deficient mutant following a step-up of ciprofloxacin. Our work highlights that bacterial cells show a delayed growth rate response following fluoroquinolone exposure. Most importantly, our model explains why the response is delayed: it takes many doubling times to fragment the DNA sufficiently to inhibit gene expression. We also show that the dynamical response is controlled by the timescale of DNA replication and gyrase binding/unbinding to the DNA rather than by the SOS response, challenging the accepted view. Our work highlights the importance of including detailed biophysical processes in biochemical-systems models to quantitatively predict the bacterial response to antibiotics.
Keywords: antibiotics; computer modeling; fluoroquinolones; mechanisms of action.
Copyright © 2020 American Society for Microbiology.
Figures


References
-
- Ena J, del Mar López-Perezagua M, Martínez-Peinado C, de los Angeles Cia-Barrio M, Ruíz-López I, 1998. Emergence of ciprofloxacin resistance in Escherichia coli isolates after widespread use of fluoroquinolones. Diagn Microbiol Infect Dis 30:103–107. doi: 10.1016/S0732-8893(97)00216-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

