Preclinical Evaluation of Acylhydrazone SB-AF-1002 as a Novel Broad-Spectrum Antifungal Agent
- PMID: 32601165
- PMCID: PMC7449198
- DOI: 10.1128/AAC.00946-20
Preclinical Evaluation of Acylhydrazone SB-AF-1002 as a Novel Broad-Spectrum Antifungal Agent
Abstract
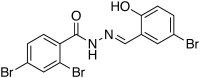
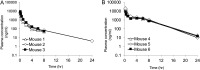
The incidence of invasive fungal infections is rising due to the increase in susceptible populations. Current clinically available drugs have therapeutic limitations due to toxicity, a narrow spectrum of activity, and, more importantly, the consistent rise of fungal species that are intrinsically resistant or that develop resistance due to prolonged therapy. Thus, there is an urgent need for new broad-spectrum antifungal agents with low toxicity and a novel mechanism of action. We previously reported a new class of potent antifungal compounds, acylhydrazones, that target the fungal sphingolipid pathway. Based upon our initial lead molecules, (E)-N'-(5-bromo-2-hydroxybenzylidene)-2-methylbenzohydrazide and D13, we performed a structure-activity relationship study, synthesizing ca. 300 new compounds. Of these, 5 compounds were identified to be the most promising for further studies, based on their broad-spectrum activity and low toxicity in mammalian cells lines. Among these top 5 lead compounds, we report here the impressive in vivo activity of 2,4-dibromo-N'-(5-bromo-2-hydroxybenzylidene)benzohydrazide (SB-AF-1002) in several models of systemic fungal infection. Our data show that SB-AF-1002 is efficacious and outperforms current standard-of-care drugs in models of invasive fungal infections, such as cryptococcosis, candidiasis, and aspergillosis. Specifically, animals treated with SB-AF-1002 not only survived the infection but also showed a clearing of fungal cells from key organs. Moreover, SB-AF-1002 was very effective in an aspergillosis model as a prophylactic therapy. SB-AF-1002 also displayed acceptable pharmacokinetic properties in mice, similar to those of the parent compound, D13. These results clearly indicate that our novel acylhydrazones constitute a new class of highly potent and efficacious antifungal agents which warrant further development for the treatment of invasive fungal infections.
Keywords: Aspergillus; Candida; Cryptococcus; acylhydrazone; antifungal agents; invasive fungal infections.
Copyright © 2020 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

