The FDA-Approved Drug Nelfinavir Inhibits Lytic Cell-Free but Not Cell-Associated Nonlytic Transmission of Human Adenovirus
- PMID: 32601166
- PMCID: PMC7449217
- DOI: 10.1128/AAC.01002-20
The FDA-Approved Drug Nelfinavir Inhibits Lytic Cell-Free but Not Cell-Associated Nonlytic Transmission of Human Adenovirus
Abstract
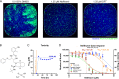
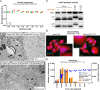
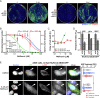
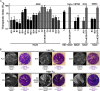
Adenoviruses (AdVs) are prevalent and give rise to chronic and recurrent disease. Human AdV (HAdV) species B and C, such as HAdV-C2, -C5, and -B14, cause respiratory disease and constitute a health threat for immunocompromised individuals. HAdV-Cs are well known for lysing cells owing to the E3 CR1-β-encoded adenovirus death protein (ADP). We previously reported a high-throughput image-based screening framework and identified an inhibitor of HAdV-C2 multiround infection, nelfinavir mesylate. Nelfinavir is the active ingredient of Viracept, an FDA-approved inhibitor of human immunodeficiency virus (HIV) aspartyl protease that is used to treat AIDS. It is not effective against single-round HAdV infections. Here, we show that nelfinavir inhibits lytic cell-free transmission of HAdV, indicated by the suppression of comet-shaped infection foci in cell culture. Comet-shaped foci occur upon convection-based transmission of cell-free viral particles from an infected cell to neighboring uninfected cells. HAdV lacking ADP was insensitive to nelfinavir but gave rise to comet-shaped foci, indicating that ADP enhances but is not required for cell lysis. This was supported by the notion that HAdV-B14 and -B14p1 lacking ADP were highly sensitive to nelfinavir, although HAdV-A31, -B3, -B7, -B11, -B16, -B21, -D8, -D30, and -D37 were less sensitive. Conspicuously, nelfinavir uncovered slow-growing round HAdV-C2 foci, independent of neutralizing antibodies in the medium, indicative of nonlytic cell-to-cell transmission. Our study demonstrates the repurposing potential of nelfinavir with postexposure efficacy against different HAdVs and describes an alternative nonlytic cell-to-cell transmission mode of HAdV.
Keywords: adenovirus death protein; antiviral agents; cell lysis; compound screening; drug repurposing; fluorescence imaging; membrane rupture; oncolytic virus; plaque assay; virus transmission.
Copyright © 2020 Georgi et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

