OGT suppresses S6K1-mediated macrophage inflammation and metabolic disturbance
- PMID: 32601203
- PMCID: PMC7368321
- DOI: 10.1073/pnas.1916121117
OGT suppresses S6K1-mediated macrophage inflammation and metabolic disturbance
Abstract
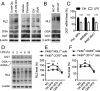
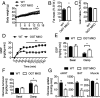
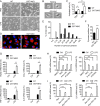
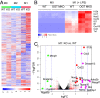
Enhanced inflammation is believed to contribute to overnutrition-induced metabolic disturbance. Nutrient flux has also been shown to be essential for immune cell activation. Here, we report an unexpected role of nutrient-sensing O-linked β-N-acetylglucosamine (O-GlcNAc) signaling in suppressing macrophage proinflammatory activation and preventing diet-induced metabolic dysfunction. Overnutrition stimulates an increase in O-GlcNAc signaling in macrophages. O-GlcNAc signaling is down-regulated during macrophage proinflammatory activation. Suppressing O-GlcNAc signaling by O-GlcNAc transferase (OGT) knockout enhances macrophage proinflammatory polarization, promotes adipose tissue inflammation and lipolysis, increases lipid accumulation in peripheral tissues, and exacerbates tissue-specific and whole-body insulin resistance in high-fat-diet-induced obese mice. OGT inhibits macrophage proinflammatory activation by catalyzing ribosomal protein S6 kinase beta-1 (S6K1) O-GlcNAcylation and suppressing S6K1 phosphorylation and mTORC1 signaling. These findings thus identify macrophage O-GlcNAc signaling as a homeostatic mechanism maintaining whole-body metabolism under overnutrition.
Keywords: RNA sequencing; immunometabolism; knockout mice; metabolic homeostasis.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Shulman G. I., Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 2237–2238 (2014). - PubMed
-
- Kahn S. E., Hull R. L., Utzschneider K. M., Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006). - PubMed
-
- Gregor M. F., Hotamisligil G. S., Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011). - PubMed
-
- Reilly S. M., Saltiel A. R., Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM122078/GM/NIGMS NIH HHS/United States
- R01 DK089098/DK/NIDDK NIH HHS/United States
- R01 DK113984/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R21 CA209848/CA/NCI NIH HHS/United States
- P30 AR072582/AR/NIAMS NIH HHS/United States
- K99 CA215315/CA/NCI NIH HHS/United States
- R01 DK102648/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
- R01 DK116774/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- P01 DK057751/DK/NIDDK NIH HHS/United States
- U01 DA045300/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

