Differential Impacts of Repeated Sampling on Odor Representations by Genetically-Defined Mitral and Tufted Cell Subpopulations in the Mouse Olfactory Bulb
- PMID: 32601245
- PMCID: PMC7406285
- DOI: 10.1523/JNEUROSCI.0258-20.2020
Differential Impacts of Repeated Sampling on Odor Representations by Genetically-Defined Mitral and Tufted Cell Subpopulations in the Mouse Olfactory Bulb
Abstract
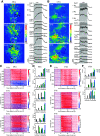
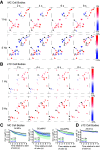
Sniffing, the active control of breathing beyond passive respiration, is used by mammals to modulate olfactory sampling. Sniffing allows animals to make odor-guided decisions within ∼200 ms, but animals routinely engage in bouts of high-frequency sniffing spanning several seconds; the impact of such repeated odorant sampling on odor representations remains unclear. We investigated this question in the mouse olfactory bulb (OB), where mitral and tufted cells (MTCs) form parallel output streams of odor information processing. To test the impact of repeated odorant sampling on MTC responses, we used two-photon imaging in anesthetized male and female mice to record activation of MTCs while precisely varying inhalation frequency. A combination of genetic targeting and viral expression of GCaMP6 reporters allowed us to access mitral cell (MC) and superficial tufted cell (sTC) subpopulations separately. We found that repeated odorant sampling differentially affected responses in MCs and sTCs, with MCs showing more diversity than sTCs over the same time period. Impacts of repeated sampling among MCs included both increases and decreases in excitation, as well as changes in response polarity. Response patterns across simultaneously-imaged MCs reformatted over time, with representations of different odorants becoming more distinct. Individual MCs responded differentially to changes in inhalation frequency, whereas sTC responses were more uniform over time and across frequency. Our results support the idea that MCs and TCs comprise functionally distinct pathways for odor information processing, and suggest that the reformatting of MC odor representations by high-frequency sniffing may serve to enhance the discrimination of similar odors.SIGNIFICANCE STATEMENT Repeated sampling of odorants during high-frequency respiration (sniffing) is a hallmark of active odorant sampling by mammals; however, the adaptive function of this behavior remains unclear. We found distinct effects of repeated sampling on odor representations carried by the two main output channels from the mouse olfactory bulb (OB), mitral and tufted cells (MTCs). Mitral cells (MCs) showed more diverse changes in response patterns over time as compared with tufted cells (TCs), leading to odorant representations that were more distinct after repeated sampling. These results support the idea that MTCs contribute different aspects to encoding odor information, and they indicate that MCs (but not TCs) may play a primary role in the modulation of olfactory processing by sampling behavior.
Keywords: coding; in vivo; mitral cell; odor; sniffing; two-photon imaging.
Copyright © 2020 the authors.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
