N-Wasp Regulates Oligodendrocyte Myelination
- PMID: 32601246
- PMCID: PMC7406274
- DOI: 10.1523/JNEUROSCI.0912-20.2020
N-Wasp Regulates Oligodendrocyte Myelination
Erratum in
-
Erratum: Katanov et al., "N-Wasp Regulates Oligodendrocyte Myelination".J Neurosci. 2024 May 1;44(18):e0612242024. doi: 10.1523/JNEUROSCI.0612-24.2024. J Neurosci. 2024. PMID: 38627093 Free PMC article. No abstract available.
Abstract
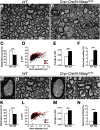
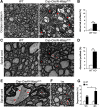
Oligodendrocyte myelination depends on actin cytoskeleton rearrangement. Neural Wiskott-Aldrich syndrome protein(N-Wasp) is an actin nucleation factor that promotes polymerization of branched actin filaments. N-Wasp activity is essential for myelin membrane wrapping by Schwann cells, but its role in oligodendrocytes and CNS myelination remains unknown. Here we report that oligodendrocytes-specific deletion of N-Wasp in mice of both sexes resulted in hypomyelination (i.e., reduced number of myelinated axons and thinner myelin profiles), as well as substantial focal hypermyelination reflected by the formation of remarkably long myelin outfolds. These myelin outfolds surrounded unmyelinated axons, neuronal cell bodies, and other myelin profiles. The latter configuration resulted in pseudo-multimyelin profiles that were often associated with axonal detachment and degeneration throughout the CNS, including in the optic nerve, corpus callosum, and the spinal cord. Furthermore, developmental analysis revealed that myelin abnormalities were already observed during the onset of myelination, suggesting that they are formed by aberrant and misguided elongation of the oligodendrocyte inner lip membrane. Our results demonstrate that N-Wasp is required for the formation of normal myelin in the CNS. They also reveal that N-Wasp plays a distinct role in oligodendrocytes compared with Schwann cells, highlighting a difference in the regulation of actin dynamics during CNS and PNS myelination.SIGNIFICANCE STATEMENT Myelin is critical for the normal function of the nervous system by facilitating fast conduction of action potentials. During the process of myelination in the CNS, oligodendrocytes undergo extensive morphological changes that involve cellular process extension and retraction, axonal ensheathment, and myelin membrane wrapping. Here we present evidence that N-Wasp, a protein regulating actin filament assembly through Arp2/3 complex-dependent actin nucleation, plays a critical role in CNS myelination, and its absence leads to several myelin abnormalities. Our data provide an important step into the understanding of the molecular mechanisms underlying CNS myelination.
Keywords: Schwann cell; actin dynamics; axon-glia; myelin; oligodendrocyte.
Copyright © 2020 the authors.
Figures



Similar articles
-
N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination.Glia. 2007 Jun;55(8):844-58. doi: 10.1002/glia.20505. Glia. 2007. PMID: 17405146
-
N-WASP is required for membrane wrapping and myelination by Schwann cells.J Cell Biol. 2011 Jan 24;192(2):243-50. doi: 10.1083/jcb.201010013. J Cell Biol. 2011. PMID: 21263026 Free PMC article.
-
mTORC2 Loss in Oligodendrocyte Progenitor Cells Results in Regional Hypomyelination in the Central Nervous System.J Neurosci. 2023 Jan 25;43(4):540-558. doi: 10.1523/JNEUROSCI.0010-22.2022. Epub 2022 Dec 2. J Neurosci. 2023. PMID: 36460463 Free PMC article.
-
The oligodendrocyte growth cone and its actin cytoskeleton: A fundamental element for progenitor cell migration and CNS myelination.Glia. 2020 Jul;68(7):1329-1346. doi: 10.1002/glia.23735. Epub 2019 Nov 7. Glia. 2020. PMID: 31696982 Free PMC article. Review.
-
The cell biology of CNS myelination.Curr Opin Neurobiol. 2016 Aug;39:93-100. doi: 10.1016/j.conb.2016.04.013. Epub 2016 May 3. Curr Opin Neurobiol. 2016. PMID: 27152449 Free PMC article. Review.
Cited by
-
CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation.Noncoding RNA. 2022 Jun 6;8(3):42. doi: 10.3390/ncrna8030042. Noncoding RNA. 2022. PMID: 35736639 Free PMC article.
-
Tuftelin1 drives experimental pulmonary fibrosis progression by facilitating stress fiber assembly.Respir Res. 2023 Dec 17;24(1):318. doi: 10.1186/s12931-023-02633-w. Respir Res. 2023. PMID: 38105232 Free PMC article.
-
Daam2 Regulates Myelin Structure and the Oligodendrocyte Actin Cytoskeleton through Rac1 and Gelsolin.J Neurosci. 2022 Mar 2;42(9):1679-1691. doi: 10.1523/JNEUROSCI.1517-21.2022. Epub 2022 Jan 31. J Neurosci. 2022. PMID: 35101966 Free PMC article.
-
FBXW7 regulates MYRF levels to control myelin capacity and homeostasis in the adult central nervous system.Nat Commun. 2025 Aug 21;16(1):7822. doi: 10.1038/s41467-025-62715-9. Nat Commun. 2025. PMID: 40841354 Free PMC article.
-
A Glance at the Molecules That Regulate Oligodendrocyte Myelination.Curr Issues Mol Biol. 2022 May 15;44(5):2194-2216. doi: 10.3390/cimb44050149. Curr Issues Mol Biol. 2022. PMID: 35678678 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
