Associations of oxygenated hemoglobin with disease burden and prognosis in stable COPD: Results from COSYCONET
- PMID: 32601330
- PMCID: PMC7324620
- DOI: 10.1038/s41598-020-67197-x
Associations of oxygenated hemoglobin with disease burden and prognosis in stable COPD: Results from COSYCONET
Abstract
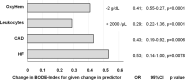
We studied whether in patients with stable COPD blood gases (BG), especially oxygenated hemoglobin (OxyHem) as a novel biomarker confer information on disease burden and prognosis and how this adds to the information provided by the comorbidity pattern and systemic inflammation. Data from 2137 patients (GOLD grades 1-4) of the baseline dataset of the COSYCONET COPD cohort were used. The associations with dyspnea, exacerbation history, BODE-Index (cut-off ≤2) and all-cause mortality over 3 years of follow-up were determined by logistic and Cox regression analyses, with sex, age, BMI and pack years as covariates. Predictive values were evaluated by ROC curves. Capillary blood gases included SaO2, PaO2, PaCO2, pH, BE and the concentration of OxyHem [haemoglobin (Hb) x fractional SaO2, g/dL] as a simple-to-measure correlate of oxygen content. Inflammatory markers were WBC, CRP, IL-6 and -8, TNF-alpha and fibrinogen, and comorbidities comprised a broad panel including cardiac and metabolic disorders. Among BG, OxyHem was associated with dyspnoea, exacerbation history, BODE-Index and mortality. Among inflammatory markers and comorbidities, only WBC and heart failure were consistently related to all outcomes. ROC analyses indicated that OxyHem provided information of a magnitude comparable to that of WBC, with optimal cut-off values of 12.5 g/dL and 8000/µL, respectively. Regarding mortality, OxyHem also carried independent, additional information, showing a hazard ratio of 2.77 (95% CI: 1.85-4.15, p < 0.0001) for values <12.5 g/dL. For comparison, the hazard ratio for WBC > 8000/µL was 2.33 (95% CI: 1.60-3.39, p < 0.0001). In stable COPD, the concentration of oxygenated hemoglobin provided additional information on disease state, especially mortality risk. OxyHem can be calculated from hemoglobin concentration and oxygen saturation without the need for the measurement of PaO2. It thus appears well suited for clinical use with minimal equipment, especially for GPs.
Conflict of interest statement
F.C. Trudzinski, R.A. Jörres. Kahnert, C. Herr, C. Kellerer, A. Omlor, S. Fähndrich, H. Watz, B. Waschki, B. Jany, S. Söhler, F. Biertz, and F. Herth have nothing to disclose with regard to this study. R. Bals reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from GlaxoSmithKline, personal fees from Grifols, grants and personal fees from Novartis, personal fees from CSL Behring, grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), grants from Sander Stiftung, grants from Schwiete Stiftung, grants from Krebshilfe, grants from Mukoviszidose eV, outside the submitted work. H.-U. Kauczor received research support for imaging investigations in the COSYCONET cohort from Bayer and Siemens; as well as research support outside the scope of this study from Philips and honoraria for speakers bureau activities from Boehringer Ingelheim, MSD and Astra Zeneca. CF Vogelmeier reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, grants and personal fees from GlaxoSmithKline, grants and personal fees from Grifols, grants and personal fees from Mundipharma, grants and personal fees from Novartis, personal fees from Berlin Chemie/Menarini, personal fees from CSL Behring, personal fees from Teva, grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), personal fees from Nuvaira, personal fees from MedUpdate, outside the submitted work. P. Alter reports grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), grants from AstraZeneca GmbH, grants and non-financial support from Bayer Schering Pharma AG, grants, personal fees and non-financial support from Boehringer Ingelheim Pharma GmbH & Co. KG, grants and non-financial support from Chiesi GmbH, grants from GlaxoSmithKline, grants from Grifols Deutschland GmbH, grants from MSD Sharp & Dohme GmbH, grants and personal fees from Mundipharma GmbH, grants, personal fees and non-financial support from Novartis Deutschland GmbH, grants from Pfizer Pharma GmbH, grants from Takeda Pharma Vertrieb GmbH & Co. KG, outside the submitted work.T. Welte reports grants from German Ministry of Research and Education (BMBF), during the conduct of the study.
Figures


References
-
- Singh, D. et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J53, 10.1183/13993003.00164-2019 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

