Removal of an abluminal lining improves decellularization of human umbilical arteries
- PMID: 32601366
- PMCID: PMC7324607
- DOI: 10.1038/s41598-020-67417-4
Removal of an abluminal lining improves decellularization of human umbilical arteries
Abstract
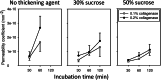
The decellularization of long segments of tubular tissues such as blood vessels may be improved by perfusing decellularization solution into their lumen. Particularly, transmural flow that may be introduced by the perfusion, if any, is beneficial to removing immunogenic cellular components in the vessel wall. When human umbilical arteries (HUAs) were perfused at a transmural pressure, however, very little transmural flow was observed. We hypothesized that a watertight lining at the abluminal surface of HUAs hampered the transmural flow and tested the hypothesis by subjecting the abluminal surface to enzyme digestion. Specifically, a highly viscous collagenase solution was applied onto the surface, thereby restricting the digestion to the surface. The localized digestion resulted in a water-permeable vessel without damaging the vessel wall. The presence of the abluminal lining and its successful removal were also supported by evidence from SEM, TEM, and mechanical testing. The collagenase-treated HUAs were decellularized with 1% sodium dodecyl sulfate (SDS) solution under either rotary agitation, simple perfusion, or pressurized perfusion. Regardless of decellularization conditions, the decellularization of HUAs was significantly enhanced after the abluminal lining removal. Particularly, complete removal of DNA was accomplished in 24 h by pressurized perfusion of the SDS solution. We conclude that the removal of the abluminal lining can improve the perfusion-assisted decellularization.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

