Cellular senescence and Alzheimer disease: the egg and the chicken scenario
- PMID: 32601397
- PMCID: PMC12548380
- DOI: 10.1038/s41583-020-0325-z
Cellular senescence and Alzheimer disease: the egg and the chicken scenario
Erratum in
-
Author Correction: Cellular senescence and Alzheimer disease: the egg and the chicken scenario.Nat Rev Neurosci. 2020 Oct;21(10):587. doi: 10.1038/s41583-020-0366-3. Nat Rev Neurosci. 2020. PMID: 32792667
Abstract
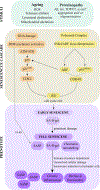
Globally, 50 million people live with dementia, with Alzheimer disease (AD) being responsible for two-thirds of the total cases. As ageing is the main risk factor for dementia-related neurodegeneration, changes in the timing or nature of the cellular hallmarks of normal ageing might be key to understanding the events that convert normal ageing into neurodegeneration. Cellular senescence is a candidate mechanism that might be important for this conversion. Under persistent stress, as occurs in ageing, both postmitotic cells - including neurons - and proliferative cells - such as astrocytes and microglia, among others - can engender a state of chronic cellular senescence that is characterized by the secretion of pro-inflammatory molecules that promote the functional decline of tissues and organs. Ablation of senescent cells has been postulated as a promising therapeutic venue to target the ageing phenotype and, thus, prevent or mitigate ageing-related diseases. However, owing to a lack of evidence, it is not possible to label cellular senescence as a cause or a consequence of neurodegeneration. This Review examines cellular senescence in the context of ageing and AD, and discusses which of the processes - cellular senescence or AD - might come first.
Figures


References
-
- Wortmann M World Alzheimer report 2014: Dementia and risk reduction. Alzheimer’s & Dementia vol. 11 P837 (2015).
-
- Winblad B et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 15, 455–532 (2016). - PubMed
-
- Ritchie K & Lovestone S The dementias. The Lancet vol. 360 1759–1766 (2002). - PubMed
-
- Hou Y et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

