AAV8 Ins1-Cre can produce efficient β-cell recombination but requires consideration of off-target effects
- PMID: 32601405
- PMCID: PMC7324556
- DOI: 10.1038/s41598-020-67136-w
AAV8 Ins1-Cre can produce efficient β-cell recombination but requires consideration of off-target effects
Abstract
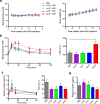
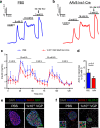
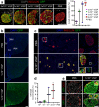
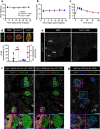
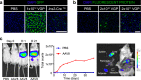
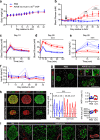
In vivo genetic manipulation is used to study the impact of gene deletion or re-expression on β-cell function and organism physiology. Cre-LoxP is a system wherein LoxP sites flanking a gene are recognized by Cre recombinase. Cre transgenic mice are the most prevalent technology used to deliver Cre but many models have caveats of off-target recombination, impaired β-cell function, and high cost of animal production. Inducible estrogen receptor conjugated Cre models face leaky recombination and confounding effects of tamoxifen. As an alternative, we characterize an adeno associated virus (AAV) with a rat insulin 1 promoter driving Cre recombinase (AAV8 Ins1-Cre) that is economical and rapid to implement, and has limited caveats. Intraperitoneal AAV8 Ins1-Cre produced efficient β-cell recombination, alongside some hepatic, exocrine pancreas, α-cell, δ-cell, and hypothalamic recombination. Delivery of lower doses via the pancreatic duct retained good rates of β-cell recombination and limited rates of off-target recombination. Unlike inducible Cre in transgenic mice, AAV8 Ins1-Cre required no tamoxifen and premature recombination was avoided. We demonstrate the utility of this technology by inducing hyperglycemia in inducible insulin knockout mice (Ins1-/-;Ins2f/f). AAV-mediated expression of Cre in β-cells provides an effective alternative to transgenic approaches for inducible knockout studies.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

