PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab
- PMID: 32601673
- PMCID: PMC8311572
- DOI: 10.1093/ndt/gfaa066
PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab
Abstract
Background: The primary challenge of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) patient care is the early detection of relapses to prevent organ damage and increase survival. Potential biomarkers for relapses are ANCA and B cells, but their predictive value is a matter of debate. Therefore this study investigated how ANCA and B-cell status related to relapses in AAV patients treated with rituximab (RTX) as remission induction (RI).
Methods: This single-centre cohort study identified 110 ANCA-positive AAV patients treated with RTX between 2006 and 2018. Serial ANCA, CD19+ B-cell status and relapses were assessed >2 years.
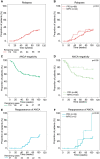
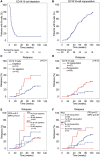
Results: Patients (31/110) relapsed within 2 years after RTX RI treatment. Patients who achieved and maintained PR3-ANCA negativity (n = 29) had few relapses (3%), while persistent proteinase 3 (PR3)-ANCA positivity (n = 49) and reappearance of PR3-ANCAs (n = 10) associated significantly with more relapses (37%, P = 0.002 and 50%, P = 0.002). Patients with incomplete B-cell depletion (n = 11) had significantly more relapses (54%) as compared with patients with B-cell depletion [n = 76 (26%), P = 0.02]. Also, patients with repopulation of B cells (n = 58) had significantly more relapses (41%) as compared with patients without B-cell repopulation [n = 27 (15%), P = 0.03]. Overall, the absence of PR3- or myeloperoxidase (MPO)-ANCA positivity was highly predictive for remaining relapse-free. In PR3-ANCA-positive patients, 96% of the relapses occurred with persistent or reappearance of PR3-ANCAs and 81% with B-cell repopulation. In MPO-ANCA-positive patients, all relapses were restricted to patients with persistent MPO-ANCAs and B-cell repopulation.
Conclusions: Upon RI treatment with RTX in AAV patients, ANCA and B-cell status were predictive of the majority of relapses and specifically their absence strongly predicted a relapse-free status. Therefore the implementation of ANCA and B-cell monitoring could guide therapeutic decision-making to prevent relapses in AAV patients treated with RTX.
Keywords: ANCA; biomarkers; crescentic glomerulonephritis; immunomonitoring; rituximab; vasculitis.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


References
-
- Jennette JC, Falk RJ.. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014; 10: 463–473 - PubMed
-
- Yates M, Watts RA, Bajema IM. et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016; 75: 1583–1594 - PubMed
-
- Jones RB, Tervaert JW, Hauser T. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

